Influence of glutathione S-transferase gene polymorphisms on busulfan pharmacokinetics and outcome of hematopoietic stem-cell transplantation in thalassemia pediatric patients
- PMID: 26691424
- PMCID: PMC4777888
- DOI: 10.1038/bmt.2015.321
Influence of glutathione S-transferase gene polymorphisms on busulfan pharmacokinetics and outcome of hematopoietic stem-cell transplantation in thalassemia pediatric patients
Abstract
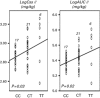
Hematopoietic stem-cell transplantation (HSCT) is currently the only curative therapeutic option for the treatment of thalassemia. In spite of the high cure rate, HSCT can lead to life-threatening adverse events in some patients. Busulfan (Bu) is a key component of the conditioning regimen prior to HSCT. Inter-individual differences in Bu pharmacokinetics (PK) are hypothesized to influence Bu efficacy and toxicity. Since Bu is mainly metabolized by glutathione S-transferase (GST), we investigated the relationship of GSTA1 and GSTM1 genotypes with first-dose PK and HSCT outcomes in 44 children with thalassemia intermedia and thalassemia major. All children received a myeloablative conditioning regimen with IV Bu. Association analysis revealed a relationship between GSTA169C>T (or haplotype *A/*B) and first Bu dose PK that was dependent on sex and Pesaro risk classification (PRC). Among female patients and patients with PRC I-II, homozygous individuals for the GSTA1T-69 allele defining haplotype *B, had higher Bu exposure and lower clearance (P⩽0.01). Association with HSCT outcomes showed that patients with the GSTM1 null genotypes had higher occurrence of regimen-related toxicity (P=0.01). These results suggest that GST genotypes could be useful to tailor the first Bu dose accordingly to improve HSCT outcome.
Figures




Similar articles
-
Glutathione S-transferase gene variations influence BU pharmacokinetics and outcome of hematopoietic SCT in pediatric patients.Bone Marrow Transplant. 2013 Jul;48(7):939-46. doi: 10.1038/bmt.2012.265. Epub 2013 Jan 7. Bone Marrow Transplant. 2013. PMID: 23292236 Clinical Trial.
-
Influence of glutathione S-transferase A1, P1, M1, T1 polymorphisms on oral busulfan pharmacokinetics in children with congenital hemoglobinopathies undergoing hematopoietic stem cell transplantation.Pediatr Blood Cancer. 2010 Dec 1;55(6):1172-9. doi: 10.1002/pbc.22739. Pediatr Blood Cancer. 2010. PMID: 20672371
-
Influence of GST gene polymorphisms on busulfan pharmacokinetics in children.Bone Marrow Transplant. 2010 Feb;45(2):261-7. doi: 10.1038/bmt.2009.143. Epub 2009 Jul 6. Bone Marrow Transplant. 2010. PMID: 19584821
-
Pharmacogenetic aspects of drug metabolizing enzymes in busulfan based conditioning prior to allogenic hematopoietic stem cell transplantation in children.Curr Drug Metab. 2014 Mar;15(3):251-64. doi: 10.2174/1389200215666140202214012. Curr Drug Metab. 2014. PMID: 24524663 Review.
-
Effect of glutathione S-transferase genetic polymorphisms on busulfan pharmacokinetics and veno-occlusive disease in hematopoietic stem cell transplantation: A meta-analysis.Basic Clin Pharmacol Toxicol. 2019 Jun;124(6):691-703. doi: 10.1111/bcpt.13185. Epub 2018 Dec 25. Basic Clin Pharmacol Toxicol. 2019. PMID: 30511436
Cited by
-
Total Body Irradiation Forever? Optimising Chemotherapeutic Options for Irradiation-Free Conditioning for Paediatric Acute Lymphoblastic Leukaemia.Front Pediatr. 2021 Dec 10;9:775485. doi: 10.3389/fped.2021.775485. eCollection 2021. Front Pediatr. 2021. PMID: 34956984 Free PMC article. Review.
-
Pharmacometabolomics for predicting variable busulfan exposure in paediatric haematopoietic stem cell transplantation patients.Sci Rep. 2017 May 10;7(1):1711. doi: 10.1038/s41598-017-01861-7. Sci Rep. 2017. PMID: 28490733 Free PMC article.
-
Personalized pharmacokinetic targeting with busulfan in allogeneic hematopoietic stem cell transplantation in infants with acute lymphoblastic leukemia.Int J Hematol. 2019 Sep;110(3):355-363. doi: 10.1007/s12185-019-02684-0. Epub 2019 Jun 14. Int J Hematol. 2019. PMID: 31201644 Clinical Trial.
-
The analysis of GSTA1 promoter genetic and functional diversity of human populations.Sci Rep. 2021 Mar 3;11(1):5038. doi: 10.1038/s41598-021-83996-2. Sci Rep. 2021. PMID: 33658540 Free PMC article.
-
Population pharmacokinetic analysis of intravenous busulfan: GSTA1 genotype is not a predictive factor of initial dose in Chinese adult patients undergoing hematopoietic stem cell transplantation.Cancer Chemother Pharmacol. 2020 Feb;85(2):293-308. doi: 10.1007/s00280-019-04001-2. Epub 2019 Dec 13. Cancer Chemother Pharmacol. 2020. PMID: 31834435
References
-
- Lucarelli G, Angelucci E Allogeneic hematopoietic stem cell transplantation for thalassemia. In: Atkinson K, Champlin R, Ritz J, Fibbe WE, Ljungman P, Brenner MK (eds). Clinical bone marrow and blood stem cell transplantation. Cambridge University Press: Cambridge, 2004, pp 929–946.
-
- Bernardo ME, Piras E, Vacca A, Giorgiani G, Zecca M, Bertaina A et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood 2012; 120: 473–476. - PubMed
-
- Mathews V, George B, Viswabandya A, Abraham A, Ahmed R, Ganapule A et al. Improved clinical outcomes of high risk beta thalassemia major patients undergoing a HLA matched related allogeneic stem cell transplant with a treosulfan based conditioning regimen and peripheral blood stem cell grafts. PLoS ONE 2013; 8: e61637. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

