Effect of HIV co-infection on adherence to a 12-week regimen of hepatitis C virus therapy with ledipasvir and sofosbuvir
- PMID: 26691547
- PMCID: PMC10624323
- DOI: 10.1097/QAD.0000000000000903
Effect of HIV co-infection on adherence to a 12-week regimen of hepatitis C virus therapy with ledipasvir and sofosbuvir
Abstract
Objective: As the treatment of hepatitis C virus (HCV) infection has evolved to directly acting antiviral agents, the impact of these directly acting antiviral-only regimens on improving adherence to HCV treatment in HIV/HCV coinfected populations has not been evaluated. The study compared adherence to ledipasvir/sofosbuvir (LDV/SOF) in HCV monoinfected and HIV/HCV coinfected individuals.
Design: Adherence was measured from participants in two phase 2 open-label studies (NCT01805882 and NCT01878799).
Methods: HCV treatment-naive, genotype 1 study individuals [HCV monoinfected participants (N = 20) and HIV/HCV coinfected participants, antiretroviral untreated (N = 13) or on combination antiretroviral therapy (N = 37)] were treated with LDV (90 mg) and SOF (400 mg) administered as one tablet once daily for 12 weeks. Adherence was measured using three tools: medication event monitoring system cap, pill count, and patient report.
Results: Participants were predominately African American (83%) and male (73%), with a median age of 59 years. Participants had prompt HCV viral load decline and high adherence rates (97 ± 0.5% by medication event monitoring system). Participant adherence decreased significantly from early (baseline week 4) as compared with late (weeks 8-12) in therapy in all three groups - HCV monoinfected (P = 0.01), HIV/HCV antiretroviral untreated (P = 0.02), and HIV/HCV antiretroviral treated participants (P = 0.01).
Conclusion: Adherence to LDV/SOF in this urban population was high and comparable between HCV monoinfected and HIV/HCV coinfected participants regardless of antiretroviral use.
Conflict of interest statement
Conflicts of interest
A.O. is an employee of Gilead Sciences, Inc. There are no other conflicts of interest.
Figures
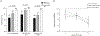
References
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–1342. - PubMed
-
- Ingiliz P, Rockstroh JK. HIV-HCV co-infection facing HCV protease inhibitor licensing: implications for clinicians. Liver Int 2012; 32:1194–1199. - PubMed
-
- Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res 2010; 85:303–315. - PubMed
-
- Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez-Tapias JM, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS 2004; 18:F27–F36. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

