Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class
- PMID: 26691591
- PMCID: PMC4686753
- DOI: 10.1371/journal.pone.0144854
Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class
Abstract
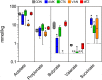
Antibiotics are frequently administered orally to treat bacterial infections not necessarily related to the gastrointestinal system. This has adverse effects on the commensal gut microbial community, as it disrupts the intricate balance between specific bacterial groups within this ecosystem, potentially leading to dysbiosis. We hypothesized that modulation of community composition and function induced by antibiotics affects intestinal integrity depending on the antibiotic administered. To address this a total of 60 Wistar rats (housed in pairs with 6 cages per group) were dosed by oral gavage with either amoxicillin (AMX), cefotaxime (CTX), vancomycin (VAN), metronidazole (MTZ), or water (CON) daily for 10-11 days. Bacterial composition, alpha diversity and caecum short chain fatty acid levels were significantly affected by AMX, CTX and VAN, and varied among antibiotic treatments. A general decrease in diversity and an increase in the relative abundance of Proteobacteria was observed for all three antibiotics. Additionally, the relative abundance of Bifidobacteriaceae was increased in the CTX group and both Lactobacillaceae and Verrucomicrobiaceae were increased in the VAN group compared to the CON group. No changes in microbiota composition or function were observed following MTZ treatment. Intestinal permeability to 4 kDa FITC-dextran decreased after CTX and VAN treatment and increased following MTZ treatment. Plasma haptoglobin levels were increased by both AMX and CTX but no changes in expression of host tight junction genes were found in any treatment group. A strong correlation between the level of caecal succinate, the relative abundance of Clostridiaceae 1 family in the caecum, and the level of acute phase protein haptoglobin in blood plasma was observed. In conclusion, antibiotic-induced changes in microbiota may be linked to alterations in intestinal permeability, although the specific interactions remain to be elucidated as changes in permeability did not always result from major changes in microbiota and vice versa.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

