Nano-Bioelectronics
- PMID: 26691648
- PMCID: PMC4867216
- DOI: 10.1021/acs.chemrev.5b00608
Nano-Bioelectronics
Abstract
Nano-bioelectronics represents a rapidly expanding interdisciplinary field that combines nanomaterials with biology and electronics and, in so doing, offers the potential to overcome existing challenges in bioelectronics. In particular, shrinking electronic transducer dimensions to the nanoscale and making their properties appear more biological can yield significant improvements in the sensitivity and biocompatibility and thereby open up opportunities in fundamental biology and healthcare. This review emphasizes recent advances in nano-bioelectronics enabled with semiconductor nanostructures, including silicon nanowires, carbon nanotubes, and graphene. First, the synthesis and electrical properties of these nanomaterials are discussed in the context of bioelectronics. Second, affinity-based nano-bioelectronic sensors for highly sensitive analysis of biomolecules are reviewed. In these studies, semiconductor nanostructures as transistor-based biosensors are discussed from fundamental device behavior through sensing applications and future challenges. Third, the complex interface between nanoelectronics and living biological systems, from single cells to live animals, is reviewed. This discussion focuses on representative advances in electrophysiology enabled using semiconductor nanostructures and their nanoelectronic devices for cellular measurements through emerging work where arrays of nanoelectronic devices are incorporated within three-dimensional cell networks that define synthetic and natural tissues. Last, some challenges and exciting future opportunities are discussed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











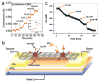

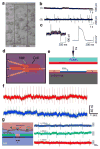



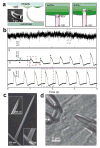
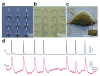





References
-
- Wang J. Glucose biosensors: 40 years of advances and challenges. Electroanal. 2001;13:983–988.
-
- Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108:814–825. - PubMed
-
- Chen C, Xie Q, Yang D, Xiao H, Fu Y, Tan Y, Yao S. Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Adv. 2013;3:4473–4491.
-
- Benabid AL. Deep Brain Stimulation for Parkinson’s Disease. Curr Opin Neurobiol. 2003;13:696–706. - PubMed
-
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

