New perspective on glycoside hydrolase binding to lignin from pretreated corn stover
- PMID: 26691693
- PMCID: PMC4683727
- DOI: 10.1186/s13068-015-0397-6
New perspective on glycoside hydrolase binding to lignin from pretreated corn stover
Abstract
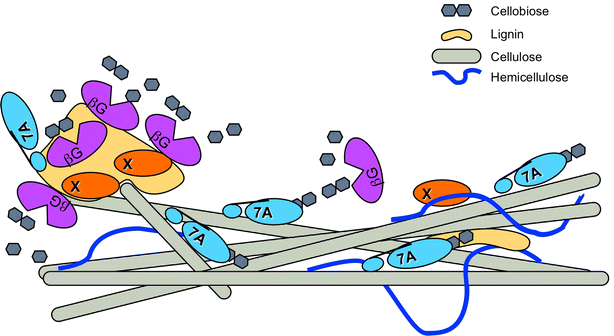
Background: Non-specific binding of cellulases to lignin has been implicated as a major factor in the loss of cellulase activity during biomass conversion to sugars. It is believed that this binding may strongly impact process economics through loss of enzyme activities during hydrolysis and enzyme recycling scenarios. The current model suggests glycoside hydrolase activities are lost though non-specific/non-productive binding of carbohydrate-binding domains to lignin, limiting catalytic site access to the carbohydrate components of the cell wall.
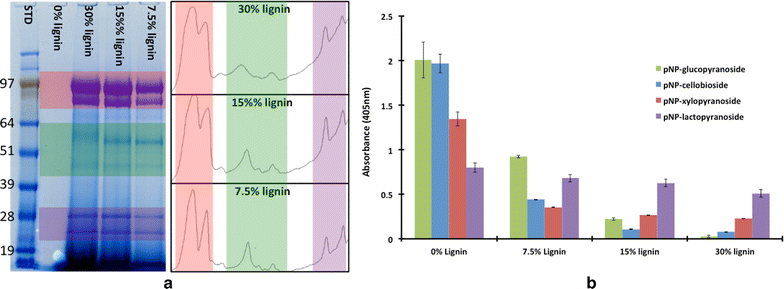
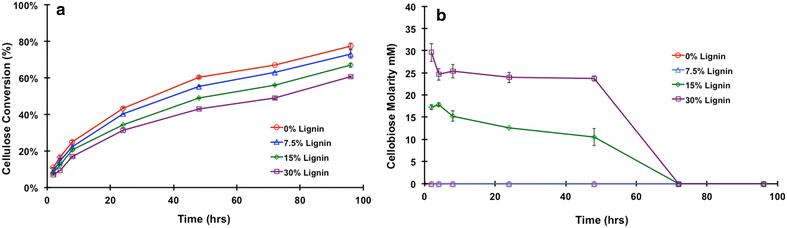
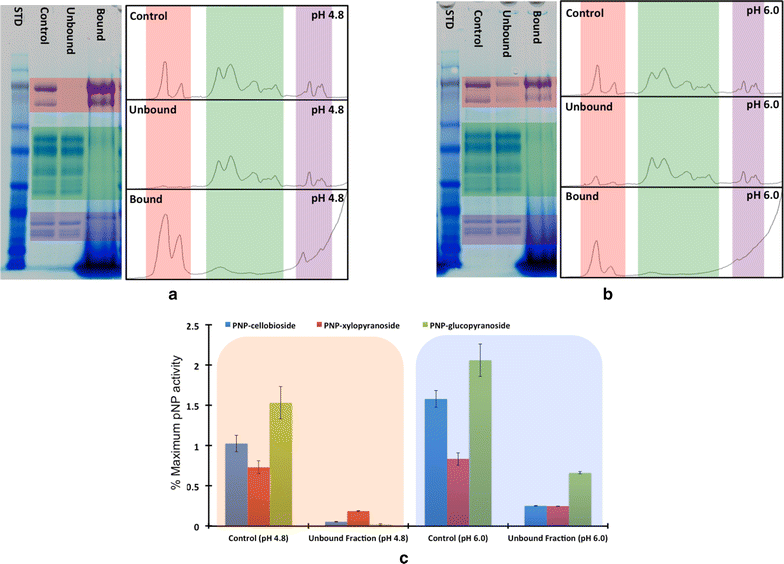
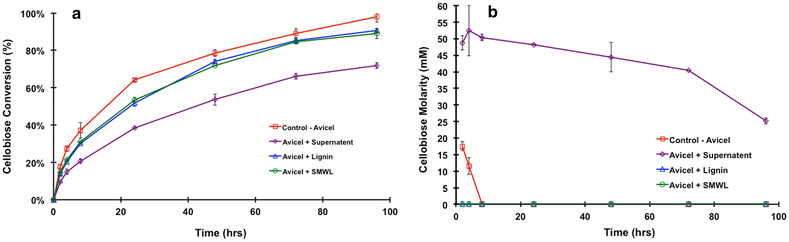
Results: In this study, we have compared component enzyme affinities of a commercial Trichoderma reesei cellulase formulation, Cellic CTec2, towards extracted corn stover lignin using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and p-nitrophenyl substrate activities to monitor component binding, activity loss, and total protein binding. Protein binding was strongly affected by pH and ionic strength. β-d-glucosidases and xylanases, which do not have carbohydrate-binding modules (CBMs) and are basic proteins, demonstrated the strongest binding at low ionic strength, suggesting that CBMs are not the dominant factor in enzyme adsorption to lignin. Despite strong adsorption to insoluble lignin, β-d-glucosidase and xylanase activities remained high, with process yields decreasing only 4-15 % depending on lignin concentration.
Conclusion: We propose that specific enzyme adsorption to lignin from a mixture of biomass-hydrolyzing enzymes is a competitive affinity where β-d-glucosidases and xylanases can displace CBM interactions with lignin. Process parameters, such as temperature, pH, and salt concentration influence the individual enzymes' affinity for lignin, and both hydrophobic and electrostatic interactions are responsible for this binding phenomenon. Moreover, our results suggest that concern regarding loss of critical cell wall degrading enzymes to lignin adsorption may be unwarranted when complex enzyme mixtures are used to digest biomass.
Keywords: Biomass; Cellulase; Enzyme binding; Glycoside hydrolase; Lignin; Pretreatment.
Figures




Similar articles
-
Lignin triggers irreversible cellulase loss during pretreated lignocellulosic biomass saccharification.Biotechnol Biofuels. 2014 Dec 13;7(1):175. doi: 10.1186/s13068-014-0175-x. eCollection 2014. Biotechnol Biofuels. 2014. PMID: 25530803 Free PMC article.
-
The adsorption and enzyme activity profiles of specific Trichoderma reesei cellulase/xylanase components when hydrolyzing steam pretreated corn stover.Enzyme Microb Technol. 2012 Mar 10;50(3):195-203. doi: 10.1016/j.enzmictec.2011.12.004. Epub 2011 Dec 26. Enzyme Microb Technol. 2012. PMID: 22305175
-
Adsorption of β-glucosidases in two commercial preparations onto pretreated biomass and lignin.Biotechnol Biofuels. 2013 Nov 25;6(1):165. doi: 10.1186/1754-6834-6-165. Biotechnol Biofuels. 2013. PMID: 24274678 Free PMC article.
-
Thermostable enzymes in lignocellulose hydrolysis.Adv Biochem Eng Biotechnol. 2007;108:121-45. doi: 10.1007/10_2007_065. Adv Biochem Eng Biotechnol. 2007. PMID: 17589813 Review.
-
Carbohydrate-binding modules facilitate the enzymatic hydrolysis of lignocellulosic biomass: Releasing reducing sugars and dissociative lignin available for producing biofuels and chemicals.Biotechnol Adv. 2023 Jul-Aug;65:108126. doi: 10.1016/j.biotechadv.2023.108126. Epub 2023 Mar 14. Biotechnol Adv. 2023. PMID: 36921877 Review.
Cited by
-
Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis.Biotechnol Biofuels. 2021 Oct 20;14(1):205. doi: 10.1186/s13068-021-02054-1. Biotechnol Biofuels. 2021. PMID: 34670604 Free PMC article. Review.
-
Synchrotron Time-Lapse Imaging of Lignocellulosic Biomass Hydrolysis: Tracking Enzyme Localization by Protein Autofluorescence and Biochemical Modification of Cell Walls by Microfluidic Infrared Microspectroscopy.Front Plant Sci. 2018 Feb 20;9:200. doi: 10.3389/fpls.2018.00200. eCollection 2018. Front Plant Sci. 2018. PMID: 29515611 Free PMC article.
-
Adding tetrahydrofuran to dilute acid pretreatment provides new insights into substrate changes that greatly enhance biomass deconstruction by Clostridium thermocellum and fungal enzymes.Biotechnol Biofuels. 2017 Nov 30;10:252. doi: 10.1186/s13068-017-0937-3. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 29213312 Free PMC article.
-
Brassinosteroid overproduction improves lignocellulose quantity and quality to maximize bioethanol yield under green-like biomass process in transgenic poplar.Biotechnol Biofuels. 2020 Jan 18;13:9. doi: 10.1186/s13068-020-1652-z. eCollection 2020. Biotechnol Biofuels. 2020. PMID: 31988661 Free PMC article.
-
Model-based optimization and scale-up of multi-feed simultaneous saccharification and co-fermentation of steam pre-treated lignocellulose enables high gravity ethanol production.Biotechnol Biofuels. 2016 Apr 18;9:88. doi: 10.1186/s13068-016-0500-7. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27096006 Free PMC article.
References
-
- Vinzant TB, Ehrman CI, Adney WS, Thomas SR, Himmel ME. Simultaneous saccharification and fermentation of pretreated hardwoods—effect of native lignin content. Appl Biochem Biotechnol. 1997;62(1):99–104. doi: 10.1007/BF02787987. - DOI
-
- Mooney CA, Mansfield SD, Touhy MG, Saddler JN. The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresour Technol. 1998;64(2):113–119. doi: 10.1016/S0960-8524(97)00181-8. - DOI
-
- Vinzant TB, Ponfick L, Nagle NJ, Ehrman CI, Reynolds JB, Himmel ME. Ssf comparison of selected woods from Southern sawmills. Appl Biochem Biotechnol. 1994;45–6:611–626. doi: 10.1007/BF02941834. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

