Tau missorting and spastin-induced microtubule disruption in neurodegeneration: Alzheimer Disease and Hereditary Spastic Paraplegia
- PMID: 26691836
- PMCID: PMC4687341
- DOI: 10.1186/s13024-015-0064-1
Tau missorting and spastin-induced microtubule disruption in neurodegeneration: Alzheimer Disease and Hereditary Spastic Paraplegia
Abstract
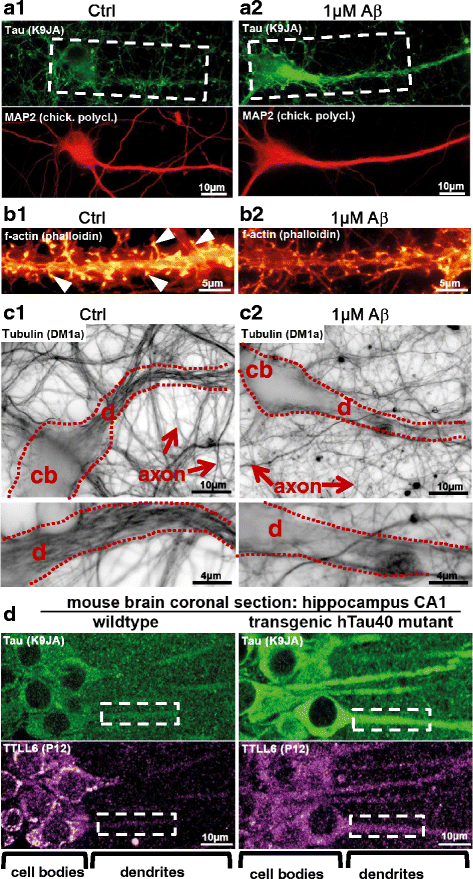
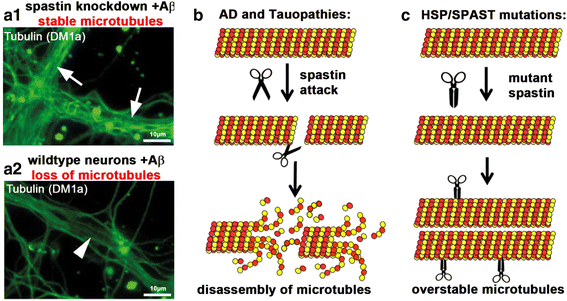
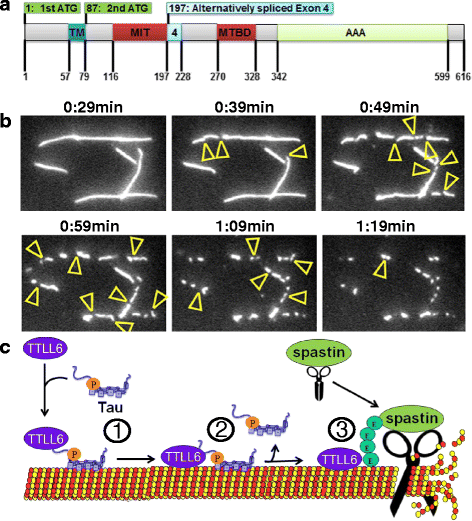
In Alzheimer Disease (AD), the mechanistic connection of the two major pathological hallmarks, namely deposition of Amyloid-beta (Aβ) in the form of extracellular plaques, and the pathological changes of the intracellular protein Tau (such as phosphorylation, missorting, aggregation), is not well understood. Genetic evidence from AD and Down Syndrome (Trisomy 21), and animal models thereof, suggests that aberrant production of Aβ is upstream of Tau aggregation, but also points to Tau as a critical effector in the pathological process. Yet, the cascade of events leading from increased levels of Aβ to Tau-dependent toxicity remains a matter of debate.Using primary neurons exposed to oligomeric forms of Aβ, we have found that Tau becomes mislocalized (missorted) into the somatodendritic compartment. Missorting of Tau correlates with loss of microtubules and downstream consequences such as loss of mature spines, loss of synaptic activity, and mislocalization of mitochondria.In this cascade, missorting of Tau induces mislocalization of TTLL6 (Tubulin-Tyrosine-Ligase-Like 6) into the dendrites. TTLL6 induces polyglutamylation of microtubules, which acts as a trigger for spastin mediated severing of dendritic microtubules. Loss of microtubules makes cells unable to maintain transport of mitochondria, which in turn results in synaptic dysfunction and loss of mature spines. These pathological changes are absent in TauKO derived primary neurons. Thus, Tau mediated mislocalization of TTLL6 and spastin activation reveals a pathological gain of function for Tau and spastin in this cellular model system of AD.In contrast, in hereditary spastic paraplegia (HSP) caused by mutations of the gene encoding spastin (spg4 alias SPAST), spastin function in terms of microtubule severing is decreased at least for the gene product of the mutated allele, resulting in overstable microtubules in disease model systems. Whether total spastin severing activity or microtubule stability in human disease is also affected is not yet clear. No human disease has been associated so far with the long-chain polyglutamylation enzyme TTLL6, or the other TTLLs (1,5,11) possibly involved.Here we review the findings supporting a role for Tau, spastin and TTLL6 in AD and other tauopathies, HSP and neurodegeneration, and summarize possible therapeutic approaches for AD and HSP.
Figures
Similar articles
-
Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin.EMBO J. 2013 Nov 13;32(22):2920-37. doi: 10.1038/emboj.2013.207. Epub 2013 Sep 24. EMBO J. 2013. PMID: 24065130 Free PMC article.
-
Repeated Exposure to Sevoflurane in Neonatal Mice Induces Cognitive and Synaptic Impairments in a TTLL6-Mediated Tubulin Polyglutamylation Manner.CNS Neurosci Ther. 2025 Apr;31(4):e70376. doi: 10.1111/cns.70376. CNS Neurosci Ther. 2025. PMID: 40202105 Free PMC article.
-
Linking amyloid-β and tau: amyloid-β induced synaptic dysfunction via local wreckage of the neuronal cytoskeleton.Neurodegener Dis. 2012;10(1-4):64-72. doi: 10.1159/000332816. Epub 2011 Dec 7. Neurodegener Dis. 2012. PMID: 22156588
-
Hereditary spastic paraplegia SPG4: what is known and not known about the disease.Brain. 2015 Sep;138(Pt 9):2471-84. doi: 10.1093/brain/awv178. Epub 2015 Jun 20. Brain. 2015. PMID: 26094131 Free PMC article. Review.
-
Lost after translation: missorting of Tau protein and consequences for Alzheimer disease.Trends Neurosci. 2014 Dec;37(12):721-32. doi: 10.1016/j.tins.2014.08.004. Epub 2014 Sep 12. Trends Neurosci. 2014. PMID: 25223701 Review.
Cited by
-
Mitochondrial and Endoplasmic Reticulum Stress Trigger Triglyceride Accumulation in Models of Parkinson's Disease Independent of Mutations in MAPT.Metabolites. 2023 Jan 9;13(1):112. doi: 10.3390/metabo13010112. Metabolites. 2023. PMID: 36677037 Free PMC article.
-
The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology.Neural Dev. 2019 Nov 9;14(1):11. doi: 10.1186/s13064-019-0134-0. Neural Dev. 2019. PMID: 31706327 Free PMC article. Review.
-
Radiosynthesis and in Vivo Evaluation of [11C]MPC-6827, the First Brain Penetrant Microtubule PET Ligand.J Med Chem. 2018 Mar 8;61(5):2118-2123. doi: 10.1021/acs.jmedchem.8b00028. Epub 2018 Feb 23. J Med Chem. 2018. PMID: 29457976 Free PMC article.
-
Tau oligomerization on microtubules in health and disease.Cytoskeleton (Hoboken). 2024 Jan;81(1):35-40. doi: 10.1002/cm.21785. Epub 2023 Sep 25. Cytoskeleton (Hoboken). 2024. PMID: 37747123 Free PMC article. No abstract available.
-
Long Non-coding RNA: Insight Into Mechanisms of Alzheimer's Disease.Front Mol Neurosci. 2022 Jan 14;14:821002. doi: 10.3389/fnmol.2021.821002. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35095418 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

