Pharmacokinetic Bioequivalence, Safety, and Immunogenicity of DMB-3111, a Trastuzumab Biosimilar, and Trastuzumab in Healthy Japanese Adult Males: Results of a Randomized Trial
- PMID: 26691837
- PMCID: PMC4746214
- DOI: 10.1007/s40259-015-0153-2
Pharmacokinetic Bioequivalence, Safety, and Immunogenicity of DMB-3111, a Trastuzumab Biosimilar, and Trastuzumab in Healthy Japanese Adult Males: Results of a Randomized Trial
Abstract
Background: DMB-3111 is a biosimilar trastuzumab drug being jointly developed by Meiji Seika Pharma (Japan) and Dong-A Socio Holdings (Korea). We investigated the bioequivalence of DMB-3111 relative to trastuzumab.
Objectives: The aim of this study was to investigate the bioequivalence between DMB-3111 and trastuzumab and the pharmacokinetic, safety, and immunogenicity of both drugs in healthy Japanese adult males.
Methods: Seventy healthy Japanese adult males were randomized 1:1 to receive either DMB-3111 or trastuzumab as a single intravenous infusion (6 mg/kg) over 90 min. Bioequivalence was assessed in terms of the pharmacokinetic parameters of both drugs. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Immunogenicity was tested using anti-drug antibody (ADA) assays.
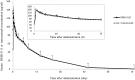
Results: The 90% confidence intervals of the treatment differences (DMB-3111 versus trastuzumab) in the mean log-transformed maximum concentration, the area under the concentration-time curves (from 0 min to the last measured value or from 0 min to infinity), mean residence time, and the terminal half-life were within the accepted range for bioequivalence [log(0.80) to log(1.25)]. The frequencies of AEs and adverse drug reactions were similar with both drugs. No ADA reactivity to DMB-3111 or trastuzumab was observed in any subject.
Conclusions: DMB-3111, a trastuzumab biosimilar, was bioequivalent to trastuzumab in terms of its pharmacokinetics and showed similar safety after a single intravenous infusion at 6 mg/kg over 90 min in healthy Japanese adult males. DMB-3111 is likely to show similar efficacy and safety profiles to trastuzumab in cancer patients (ClinicalTrials.gov #NCT02100917).
Figures


References
-
- Herceptin® for Injection. Package Insert, 23rd edn. Tokyo: Chugai Pharmaceutical Co., Ltd.; 2013.
-
- Herceptin® for Injection. Interview Form, 18th edn. Tokyo: Chugai Pharmaceutical Co., Ltd.; 2013.
-
- Guidelines for approval of biosimilars. National Institute of Health Sciences, Tokyo, Japan. http://www.nihs.go.jp/dbcb/TEXT/yakusyokuhatu-0304004.pdf.
-
- Yin D, Barker KB, Li R, Meng X, Reich SD, Ricart AD, Rudin D, Taylor CT, Zacharchuk CM, Hansson AG. A randomized phase 2 pharmacokinetic trial comparing the potential biosimilar PF-05280014 with trastuzumab in healthy volunteers (REFLECTIONS B327-01) Br J Clin Pharmacol. 2014;78:1281–1290. doi: 10.1111/bcp.12464. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

