Signalling beyond photon absorption: extracellular retinoids and growth factors modulate rod photoreceptor sensitivity
- PMID: 26691896
- PMCID: PMC4818600
- DOI: 10.1113/JP271650
Signalling beyond photon absorption: extracellular retinoids and growth factors modulate rod photoreceptor sensitivity
Abstract
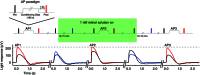
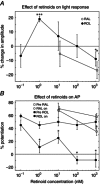
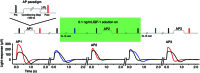
Key points: We propose that the end product of chromophore bleaching in rod photoreceptors, all-trans retinol, is part of a feedback loop that increases the sensitivity of the phototransduction cascade in rods. A previously described light-induced hypersensitivity in rods, termed adaptive potentiation, is reduced by exogenously applied all-trans retinol but not all-trans retinal. This potentiation is produced by insulin-like growth factor-1, whose binding proteins are located in the extracellular matrix, even in our isolated retina preparation after removal of the retinal pigmented epithelium. Simple modelling suggests that the light stimuli used in the present study will produce sufficient all-trans retinol within the interphotoreceptor matrix to explain the potentiation effect.
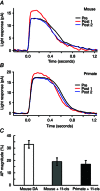
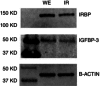
Abstract: Photoreceptors translate the absorption of photons into electrical signals for propagation through the visual system. Mammalian photoreceptor signalling has largely been studied in isolated cells, and such studies have necessarily avoided the complex environment of supportive proteins that surround the photoreceptors. The interphotoreceptor matrix (IPM) contains an array of proteins that aid in both structural maintenance and cellular homeostasis, including chromophore turnover. In signalling photon absorption, the chromophore 11-cis retinal is first isomerized to all-trans retinal, followed by conversion to all-trans retinol (ROL) for removal from the photoreceptor. Interphotoreceptor retinoid-binding protein (IRBP) is the most abundant protein in the IPM, and it promotes the removal of bleached chromophores and recycling in the nearby retinal pigment epithelium. By studying the light responses of isolated mouse retinas, we demonstrate that ROL can act as a feedback signal onto photoreceptors that influences the sensitivity of phototransduction. In addition to IRBP, the IPM also contains insulin-like growth factor-1 (IGF-1) and its associated binding proteins, although their functions have not yet been described. We demonstrate that extracellular application of physiological concentrations of IGF-1 can increase rod photoreceptor sensitivity in mammalian retinas. We also determine that chromophores and growth factors can limit the range of a newly described form of photoreceptor light adaptation. Finally, fluorescent antibodies demonstrate the presence of IRBP and IGFBP-3 in isolated retinas. A simple model of the formation and release of ROL into the extracellular space quantitatively describes this novel feedback loop.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



Similar articles
-
Interphotoreceptor retinoid-binding protein (IRBP) promotes the release of all-trans retinol from the isolated retina following rhodopsin bleaching illumination.Exp Eye Res. 2005 Oct;81(4):455-63. doi: 10.1016/j.exer.2005.03.005. Epub 2005 Jun 2. Exp Eye Res. 2005. PMID: 15935345
-
Interphotoreceptor retinoid-binding protein removes all-trans-retinol and retinal from rod outer segments, preventing lipofuscin precursor formation.J Biol Chem. 2017 Nov 24;292(47):19356-19365. doi: 10.1074/jbc.M117.795187. Epub 2017 Sep 28. J Biol Chem. 2017. PMID: 28972139 Free PMC article.
-
Confocal immunolocalization of bovine serum albumin, serum retinol-binding protein, and interphotoreceptor retinoid-binding protein in bovine retina.Mol Vis. 2006 Dec 22;12:1632-9. Mol Vis. 2006. PMID: 17200663
-
Interphotoreceptor retinoid-binding protein (IRBP). Molecular biology and physiological role in the visual cycle of rhodopsin.Mol Neurobiol. 1993 Spring;7(1):61-85. doi: 10.1007/BF02780609. Mol Neurobiol. 1993. PMID: 8318167 Review.
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
Cited by
-
Investigating the Ca2+-dependent and Ca2+-independent mechanisms for mammalian cone light adaptation.Sci Rep. 2018 Oct 26;8(1):15864. doi: 10.1038/s41598-018-34073-8. Sci Rep. 2018. PMID: 30367097 Free PMC article.
References
-
- Adler A & Edwards R (2000). Human interphotoreceptor matrix contains serum albumin and retinol‐binding protein. Exp Eye Res 70, 227–234. - PubMed
-
- Adler A & Martin K (1982). Retinol‐binding proteins in bovine interphotoreceptor matrix. Biochem Biophys Res Commun 108, 1601–1608. - PubMed
-
- Adler A & Spencer S (1991). Effect of light on endogenous ligands carried by interphotoreceptor retinoid‐binding protein. Exp Eye Res 53, 337–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

