Molecular basis of antibody binding to mucin glycopeptides in lung cancer
- PMID: 26692014
- PMCID: PMC4725460
- DOI: 10.3892/ijo.2015.3302
Molecular basis of antibody binding to mucin glycopeptides in lung cancer
Abstract
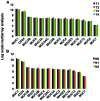
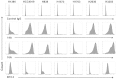
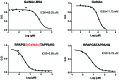
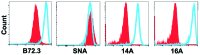
Glycopeptides bearing Tn epitopes are emerging targets for cancer diagnosis and immunotherapy. In this study, we analyzed membrane proteins containing O-glycosylated tandem repeat (TR) sequences in lung cancer patients of different types and stages, using gene microarray data in public domain. The expression of Tn and glycopeptide epitopes on the surface of lung cancer cell lines were studied by monoclonal IgG antibodies 14A, 16A, and B72.3. The binding of mAbs to synthetic glycopeptides were studied by surface plasmon resonance. Nine mucin mRNAs were found to be expressed in lung cancer patients but at similar level to healthy individuals. At protein level, a glycopeptide epitope on cancer cell surface is preferably recognized by mAb 16A, as compared to peptide-alone (14A) or sugar-alone epitopes (B72.3). 14A and 16A favor clustered TR containing more than three TR sequences, with 10-fold lower Kd than two consecutive TR. B72.3 preferrably recognized clustered sialyl-Tn displayed on MUC1 but not other O-glycoproteins, with 100-fold stronger binding when MUC1 is transfected as a sugar carrier, while the total sugar epitopes remain unchanged. These findings indicate that clusters of both TR backbones and sugars are essential for mAb binding to mucin glycopeptides. Three rules of antibody binding to mucin glycopeptides at molecular level are presented here: first, the peptide backbone of a glycopeptide is preferentially recognized by B cells through mutations in complementarity determining regions (CDRs) of B cell receptor, and the sugar-binding specificity is acquired through mutations in frame work of heavy chain; secondly, consecutive tandem repeats (TR) of peptides and glycopeptides are preferentially recognized by B cells, which favor clustered TR containing more than three TR sequences; thirdly, certain sugar-specific B cells recognize and accommodate clustered Tn and sialyl-Tn displayed on the surface of a mucin but not other membrane proteins.
Figures
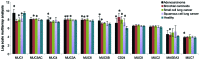
Similar articles
-
Epitopes of MUC1 Tandem Repeats in Cancer as Revealed by Antibody Crystallography: Toward Glycopeptide Signature-Guided Therapy.Molecules. 2018 May 31;23(6):1326. doi: 10.3390/molecules23061326. Molecules. 2018. PMID: 29857542 Free PMC article. Review.
-
MUC1 glycopeptide epitopes predicted by computational glycomics.Int J Oncol. 2012 Dec;41(6):1977-84. doi: 10.3892/ijo.2012.1645. Epub 2012 Sep 27. Int J Oncol. 2012. PMID: 23023583 Free PMC article.
-
Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice.Biol Chem. 2009 Jul;390(7):611-8. doi: 10.1515/BC.2009.070. Biol Chem. 2009. PMID: 19426130 Free PMC article.
-
Structure/activity studies of the anti-MUC1 monoclonal antibody C595 and synthetic MUC1 mucin-core-related peptides and glycopeptides.Biospectroscopy. 1999;5(2):79-91. doi: 10.1002/(SICI)1520-6343(1999)5:2<79::AID-BSPY2>3.0.CO;2-#. Biospectroscopy. 1999. PMID: 10217327
-
Diverse glycosylation of MUC1 and MUC2: potential significance in tumor immunity.J Biochem. 1999 Dec;126(6):975-85. doi: 10.1093/oxfordjournals.jbchem.a022565. J Biochem. 1999. PMID: 10578046 Review.
Cited by
-
Parallel Glyco-SPOT Synthesis of Glycopeptide Libraries.Cell Chem Biol. 2020 Sep 17;27(9):1207-1219.e9. doi: 10.1016/j.chembiol.2020.06.007. Epub 2020 Jun 30. Cell Chem Biol. 2020. PMID: 32610041 Free PMC article.
-
Epitopes of MUC1 Tandem Repeats in Cancer as Revealed by Antibody Crystallography: Toward Glycopeptide Signature-Guided Therapy.Molecules. 2018 May 31;23(6):1326. doi: 10.3390/molecules23061326. Molecules. 2018. PMID: 29857542 Free PMC article. Review.
-
Enhancing Cancer Vaccine Efficacy: Backbone Modification with β‑Amino Acids Alters the Stability and Immunogenicity of MUC1-Derived Glycopeptide Formulations.JACS Au. 2025 May 15;5(5):2270-2284. doi: 10.1021/jacsau.5c00224. eCollection 2025 May 26. JACS Au. 2025. PMID: 40443897 Free PMC article.
-
Immunogenicity of Del19 EGFR mutations in Chinese patients affected by lung adenocarcinoma.BMC Immunol. 2019 Nov 13;20(1):43. doi: 10.1186/s12865-019-0320-1. BMC Immunol. 2019. PMID: 31722672 Free PMC article.
-
A novel Tn antigen epitope-recognizing antibody for MUC1 predicts clinical outcome in patients with primary lung adenocarcinoma.Oncol Lett. 2021 Mar;21(3):202. doi: 10.3892/ol.2021.12463. Epub 2021 Jan 12. Oncol Lett. 2021. PMID: 33574941 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

