Characterization of a conditional interleukin-1 receptor 1 mouse mutant using the Cre/LoxP system
- PMID: 26692072
- PMCID: PMC4982085
- DOI: 10.1002/eji.201546075
Characterization of a conditional interleukin-1 receptor 1 mouse mutant using the Cre/LoxP system
Abstract
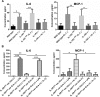
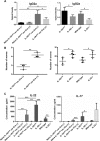
IL-1 is a key cytokine known to drive chronic inflammation and to regulate many physiological, immunological, and neuroimmunological responses via actions on diverse cell types of the body. To determine the mechanisms of IL-1 actions as part of the inflammatory response in vivo, we generated a conditional IL-1 receptor 1 (IL-1R1) mouse mutant using the Cre/LoxP system (IL-1R1(fl/fl) ). In the mutant generated, exon 5, which encodes part of the extracellular-binding region of the receptor, is flanked by LoxP sites, thereby inactivating the two previously described functional IL-1R1 gene transcripts after Cre-mediated recombination. Using keratin 14-Cre driver mice, new IL-1R1 deficient (-/-) mice were subsequently generated, in which all signaling IL-1 receptor isoforms are deleted ubiquitously. Furthermore, using vav-iCre driver mice, we deleted IL-1 receptor isoforms in the hematopoietic system. In these mice, we show that both the IL-17 and IL-22 cytokine response is reduced, when mice are challenged by the helminth Trichuris muris. We are currently crossing IL-1R1(fl/fl) mice with different Cre-expressing mice in order to study mechanisms of acute and chronic inflammatory diseases.
Keywords: Cre/loxP; IL-17; Immune regulation; Infection; Trichuris muris.
© 2015 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Glaccum, M. B. , Stocking, K. L. , Charrier, K. , Smith, J. L. , Willis, C. R. , Maliszewski, C. , Livingston, D. J. et al, Phenotypic and functional characterization of mice that lack the type I receptor for IL‐1. J. Immunol. 1997. 159: 3364–3371. - PubMed
-
- Labow, M. , Shuster, D. , Zetterstrom, M. , Nunes, P. , Terry, R. , Cullinan, E. B. , Bartfai, T. et al, Absence of IL‐1 signaling and reduced inflammatory response in IL‐1 type I receptor‐deficient mice. J. Immunol. 1997. 159: 2452–2461. - PubMed
-
- Humphreys, N. E. and Grencis, R. K. , IL‐1‐dependent, IL‐1R1‐independent resistance to gastrointestinal nematodes. Eur. J. Immunol. 2009. 39: 1036–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

