Design of a dietary intervention to assess the impact of a gluten-free diet in a population with type 1 Diabetes and Celiac Disease
- PMID: 26692164
- PMCID: PMC4687374
- DOI: 10.1186/s12876-015-0413-0
Design of a dietary intervention to assess the impact of a gluten-free diet in a population with type 1 Diabetes and Celiac Disease
Abstract
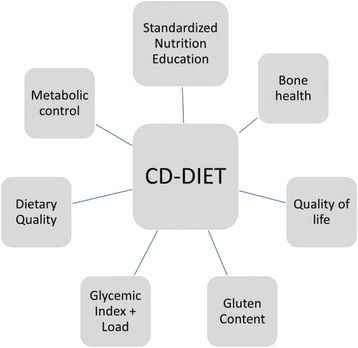
Background: Celiac Disease occurs at a 5-10 fold greater prevalence in patients with type-1 diabetes (T1D), despite this increased risk, there is limited objective evidence regarding the impact of a Gluten-Free Diet (GFD) in the large proportion of asymptomatic (30-70%) patients with both autoimmune diseases. Given the requirements and intricacies inherent to each condition, we describe the rationale and design a dietary curriculum specifically addressing the educational requirements for children and adults with CD and diabetes as part of the CD-DIET Study.
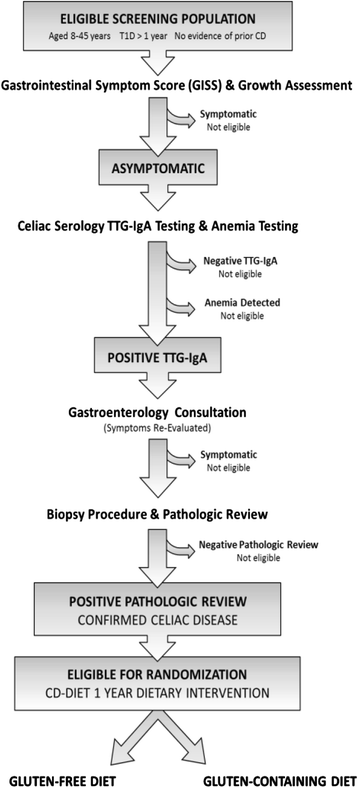
Methods and design: The CD-DIET Study (Celiac Disease and Diabetes - Dietary Intervention and Evaluation Trial) is a multicenter randomized controlled trial aimed at evaluating the safety and efficacy of a GFD in patients with asymptomatic celiac disease and T1D on key diabetes and patient-centered outcomes.
Discussion: Key dietary components of the trial include a description and evaluation of food consumption patterns including glycemic index and glycemic load, novel assessments of gluten quantification, and objective and subjective measures of GFD adherence. This dietary curriculum will establish rigorous guidelines to assess adherence and facilitate evaluation of a GFD on metabolic control, bone health and patient quality of life in patients with CD and diabetes.
Trial registration number: NCT01566110. Date of Registration: March, 2012.
Figures
Similar articles
-
The Celiac Disease and Diabetes-Dietary Intervention and Evaluation Trial (CD-DIET) protocol: a randomised controlled study to evaluate treatment of asymptomatic coeliac disease in type 1 diabetes.BMJ Open. 2015 May 11;5(5):e008097. doi: 10.1136/bmjopen-2015-008097. BMJ Open. 2015. PMID: 25968008 Free PMC article. Clinical Trial.
-
The urinary inflammatory profile in gluten free diet-adherent adolescents with type 1 diabetes and celiac disease.J Diabetes Complications. 2016 Mar;30(2):295-9. doi: 10.1016/j.jdiacomp.2015.11.020. Epub 2015 Nov 25. J Diabetes Complications. 2016. PMID: 26790575
-
Impact of a Gluten-Free Diet on Quality of Life and Health Perception in Patients With Type 1 Diabetes and Asymptomatic Celiac Disease.J Clin Endocrinol Metab. 2021 Apr 23;106(5):e1984-e1992. doi: 10.1210/clinem/dgaa977. J Clin Endocrinol Metab. 2021. PMID: 33524131 Clinical Trial.
-
Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases.Nutrients. 2020 Aug 1;12(8):2316. doi: 10.3390/nu12082316. Nutrients. 2020. PMID: 32752175 Free PMC article. Review.
-
Celiac disease: understanding the gluten-free diet.Eur J Nutr. 2017 Mar;56(2):449-459. doi: 10.1007/s00394-016-1238-5. Epub 2016 Jun 22. Eur J Nutr. 2017. PMID: 27334430 Review.
Cited by
-
Does a Gluten-Free Diet Affect BMI and Glycosylated Hemoglobin in Children and Adolescents with Type 1 Diabetes and Asymptomatic Celiac Disease? A Meta-Analysis and Systematic Review.Children (Basel). 2022 Aug 18;9(8):1247. doi: 10.3390/children9081247. Children (Basel). 2022. PMID: 36010137 Free PMC article.
-
High-Quality Gluten-Free Sponge Cakes without Sucrose: Inulin-Type Fructans as Sugar Alternatives.Foods. 2020 Nov 25;9(12):1735. doi: 10.3390/foods9121735. Foods. 2020. PMID: 33255788 Free PMC article.
-
Prevalence of haplotype DQ2/DQ8 and celiac disease in children with type 1 diabetes.Diabetol Metab Syndr. 2022 Sep 12;14(1):128. doi: 10.1186/s13098-022-00897-8. Diabetol Metab Syndr. 2022. PMID: 36096955 Free PMC article.
-
Intestinal development and homeostasis require activation and apoptosis of diet-reactive T cells.J Clin Invest. 2019 Apr 2;129(5):1972-1983. doi: 10.1172/JCI98929. eCollection 2019 Apr 2. J Clin Invest. 2019. PMID: 30939122 Free PMC article.
-
Accuracy of Serological Screening for the Diagnosis of Celiac Disease in Type 1 Diabetes Children.Medicina (Kaunas). 2023 Jul 18;59(7):1321. doi: 10.3390/medicina59071321. Medicina (Kaunas). 2023. PMID: 37512132 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical