Self-reactive IgE exacerbates interferon responses associated with autoimmunity
- PMID: 26692173
- PMCID: PMC4718782
- DOI: 10.1038/ni.3326
Self-reactive IgE exacerbates interferon responses associated with autoimmunity
Abstract
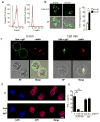
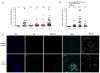
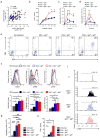
Canonically, immunoglobulin E (IgE) mediates allergic immune responses by triggering mast cells and basophils to release histamine and type 2 helper cytokines. Here we found that in human systemic lupus erythematosus (SLE), IgE antibodies specific for double-stranded DNA (dsDNA) activated plasmacytoid dendritic cells (pDCs), a type of cell of the immune system linked to viral defense, which led to the secretion of substantial amounts of interferon-α (IFN-α). The concentration of dsDNA-specific IgE found in patient serum correlated with disease severity and greatly potentiated pDC function by triggering phagocytosis via the high-affinity FcɛRI receptor for IgE, followed by Toll-like receptor 9 (TLR9)-mediated sensing of DNA in phagosomes. Our findings expand the known pathogenic mechanisms of IgE-mediated inflammation beyond those found in allergy and demonstrate that IgE can trigger interferon responses capable of exacerbating self-destructive autoimmune responses.
Figures

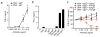

Comment in
-
Lupus pathogenesis: role of IgE autoantibodies.Cell Res. 2016 Mar;26(3):271-2. doi: 10.1038/cr.2016.12. Epub 2016 Jan 22. Cell Res. 2016. PMID: 26794873 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

