Molecular characterization of a cathepsin F-like protease in Trichinella spiralis
- PMID: 26692208
- PMCID: PMC4687129
- DOI: 10.1186/s13071-015-1270-y
Molecular characterization of a cathepsin F-like protease in Trichinella spiralis
Abstract
Background: Trichinellosis is a re-emerging infectious disease, caused by Trichinella spp. Cathepsin F belongs to cysteine protease that is a major virulence factor for parasitic helminths, and it may be a potential anti-helminth drug target and vaccine candidate. The aim of this study was to clone, express and identify a cathepsin F-like protease in Trichinella spiralis and to investigate its biochemical characteristics.
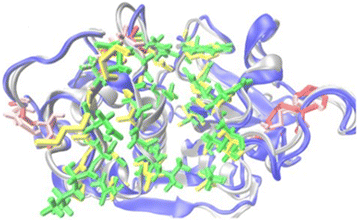
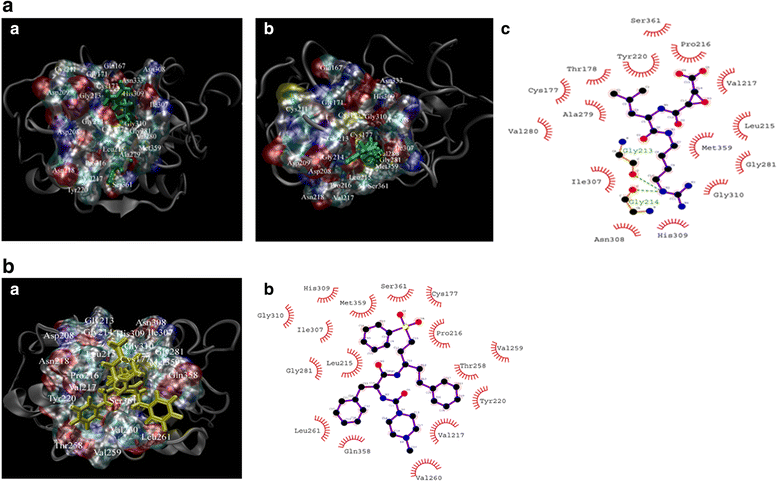
Methods: The full-length cDNA encoding a putative cathepsin F-like protease in T. spiralis, TsCF1, was cloned and its biochemical characterization and expression profile were analyzed. Transcription of TsCF1 at different developmental stages of T. spiralis was observed by RT-PCR. The recombinant TsCF1 protein was expressed by prokaryotic expression system and recombinant TsCF1 (rTsCF1) was analyzed by western blotting. And expression of TsCF1 at muscle larvae stage was performed by immunofluorescent technique. Molecular modeling of TsCF1 and its binding mode with E-64 and K11777 were analyzed. Enzyme activity and inhibitory test with E-64 as inhibitor were investigated by using Z-Phe-Arg-AMC as specific substrate.
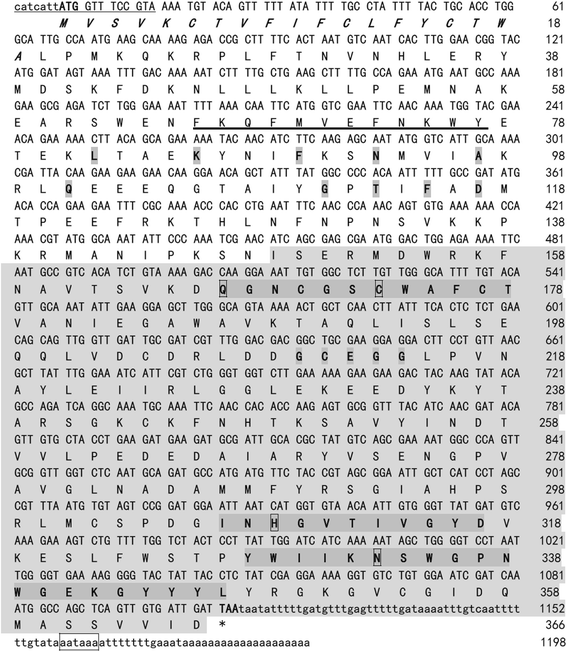
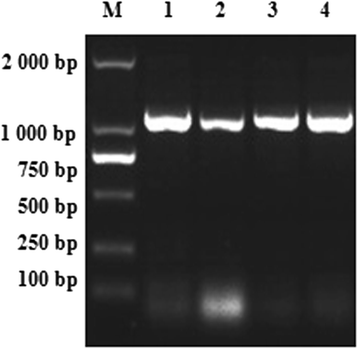
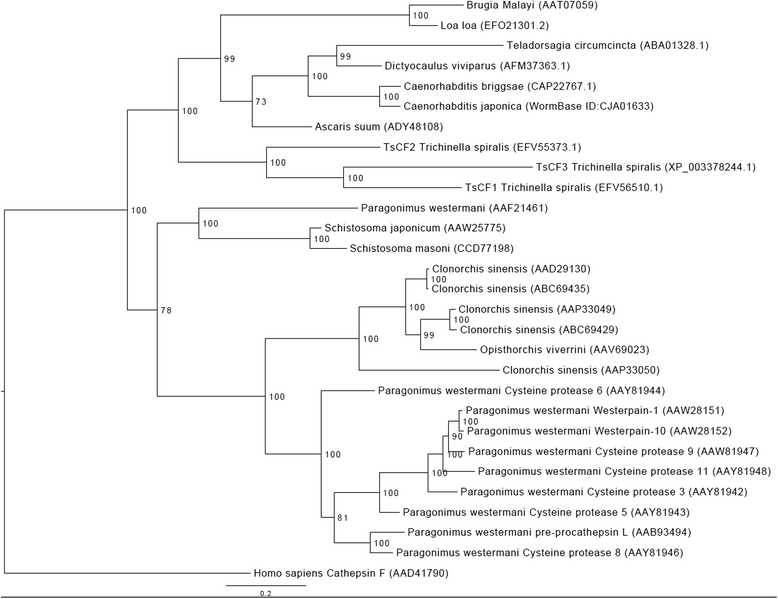
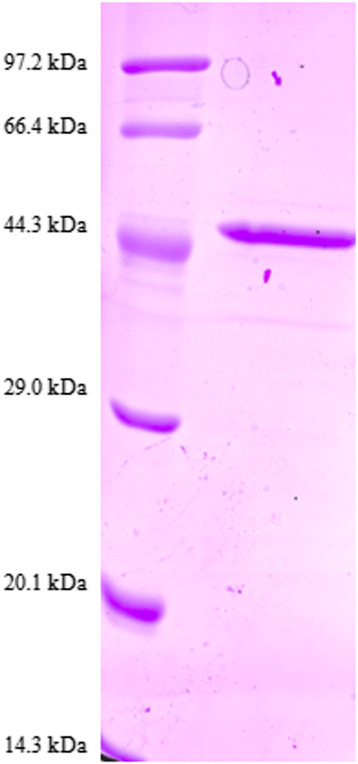
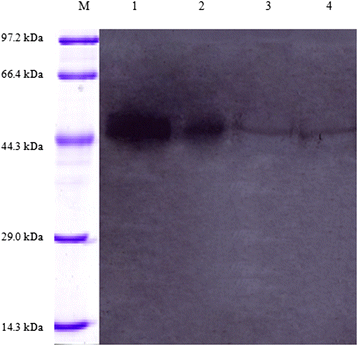
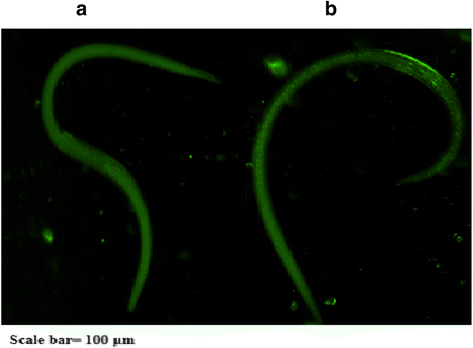
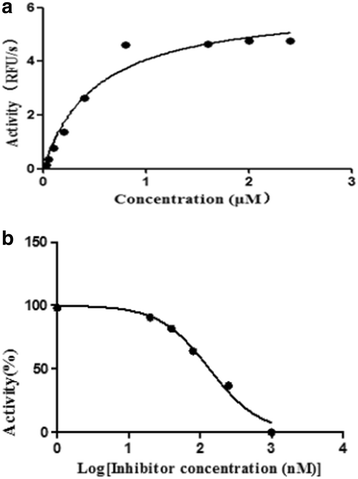
Results: Sequence analysis revealed that TsCF1 ORF encodes a protein of 366 aa with a theoretical molecular weight of 41.9 kDa and an isoelectric point of 7.46. The cysteine protease conserved active site of Cys173, His309 and Asn333 were identified and cathepsin F specific motif ERFNAQ like KLFNAQ sequence was revealed in the propeptide of TsCF1. Sequence alignment analysis revealed a higher than 40 % identity with other cathepsin F from parasitic helminth and phylogenetic analysis indicated TsCF1 located at the junction of nematode and trematode. RT-PCR revealed the gene was expressed in muscle larvae, newborn larvae and adult stages. SDS-PAGE revealed the recombinant protein was expressed with the molecular weight of 45 kDa. The purified rTsCF1 was used to immunize rabbit and the immune serum could recognize a band of about 46 kDa in soluble protein of adult, muscle larvae and ES product of muscle larvae. Immunolocalization analysis showed that TsCF1 located on the cuticle and stichosome of the muscle larvae. After renaturation rTsCF1 demonstrated substantial enzyme activity to Z-Phe-Arg-AMC substrate with the optimal pH 5.5 and this activity could be inhibited by cysteine protease inhibitor E-64. Further analysis showed the kinetic parameters of rTsCF1 to be Km = 0.5091 μM and Vmax = 6.12 RFU/s μM at pH 5.5, and the IC50 value of E64 was 135.50 ± 16.90 nM.
Conclusion: TsCF1 was expressed in all stages of T. spiralis and localized in the cuticle and stichosome. TsCF1 might play a role in the life cycle of T. spiralis and could be used as a potential vaccine candidate and drug target against T. spiralis infection.
Figures
Similar articles
-
Cloning, expression and characterization of a Trichinella spiralis serine protease gene encoding a 35.5 kDa protein.Exp Parasitol. 2013 Jun;134(2):148-54. doi: 10.1016/j.exppara.2013.03.004. Epub 2013 Mar 15. Exp Parasitol. 2013. PMID: 23501807
-
Molecular identification of Trichinella spiralis nudix hydrolase and its induced protective immunity against trichinellosis in BALB/c mice.Parasit Vectors. 2014 Dec 19;7:600. doi: 10.1186/s13071-014-0600-9. Parasit Vectors. 2014. PMID: 25522912 Free PMC article.
-
Cloning and expression of a Trichinella spiralis putative glutathione S-transferase and its elicited protective immunity against challenge infections.Parasit Vectors. 2017 Sep 29;10(1):448. doi: 10.1186/s13071-017-2384-1. Parasit Vectors. 2017. PMID: 28962639 Free PMC article.
-
Trichinella spiralis: genomic application to control a zoonotic nematode.Infect Disord Drug Targets. 2010 Oct;10(5):376-84. doi: 10.2174/187152610793180830. Infect Disord Drug Targets. 2010. PMID: 20701572 Review.
-
Advances in the sequencing of the genome of the adenophorean nematode Trichinella spiralis.Parasitology. 2008 Jul;135(8):869-80. doi: 10.1017/S0031182008004472. Parasitology. 2008. PMID: 18598573 Free PMC article. Review.
Cited by
-
Vaccination of mice with a recombinant novel cathepsin B inhibits Trichinella spiralis development, reduces the fecundity and worm burden.Parasit Vectors. 2019 Dec 11;12(1):581. doi: 10.1186/s13071-019-3833-9. Parasit Vectors. 2019. PMID: 31829230 Free PMC article.
-
Broadening Horizons: Exploring the Cathepsin Family as Therapeutic Targets for Alzheimer's Disease.Aging Dis. 2024 Jun 2;16(4):1987-2007. doi: 10.14336/AD.2024.0456. Aging Dis. 2024. PMID: 39122455 Free PMC article. Review.
-
Characterization of a novel cysteine protease in Trichinella spiralis and its role in larval intrusion, development and fecundity.Vet Res. 2021 Aug 26;52(1):113. doi: 10.1186/s13567-021-00983-1. Vet Res. 2021. PMID: 34446106 Free PMC article.
-
Comprehensive chemical profiling of Bassia indica Wight. aerial parts extract using UPLC-ESI-MS/MS, and its antiparasitic activity in Trichinella spiralis infected mice: in silico supported in vivo study.BMC Complement Med Ther. 2023 May 18;23(1):161. doi: 10.1186/s12906-023-03988-9. BMC Complement Med Ther. 2023. PMID: 37202749 Free PMC article.
-
The cysteine protease ATG4B of Trichinella spiralis promotes larval invasion into the intestine of the host.Vet Res. 2020 May 24;51(1):69. doi: 10.1186/s13567-020-00791-z. Vet Res. 2020. PMID: 32448368 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

