Arsenic trioxide reduces chemo-resistance to 5-fluorouracil and cisplatin in HBx-HepG2 cells via complex mechanisms
- PMID: 26692822
- PMCID: PMC4676851
- DOI: 10.1186/s12935-015-0269-y
Arsenic trioxide reduces chemo-resistance to 5-fluorouracil and cisplatin in HBx-HepG2 cells via complex mechanisms
Abstract
Background: Multidrug resistance is one of the major reasons chemotherapy-based treatments failed in hepatitis B virus (HBV) related hepatocellular carcinoma (HCC). Hypoxia is generally associated with tumor chemo-resistance. The aim of the study was to investigate the effect of Arsenic trioxide (As2O3) on the hypoxia-induced chemo-resistance to 5-FU or cisplatin and explored its underlying mechanism in the HBx-HepG2 cells.
Methods: MTT assay was used to examine the cell viability. Mitochondrial membrane potential (MMP) and cell cycle was examined by flow cytometry. qRT-PCR was employed to observe the mRNA expression level; and western blot assay was used to determine the protein expression level.
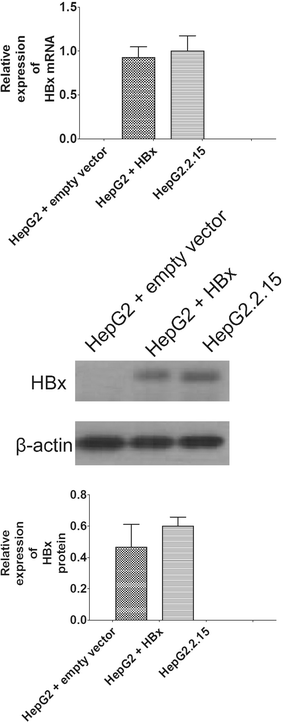
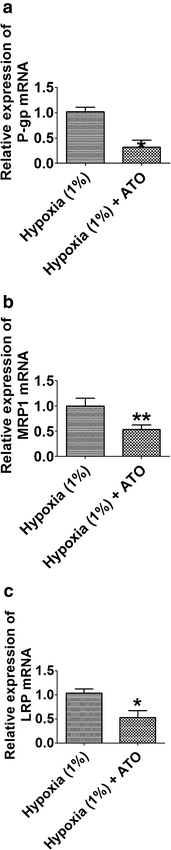
Results: Our results showed that transfection of HBx plasmid established the HBx-HepG2 cells expressing HBx, and the expression of HBx was confirmed by qRT-PCR and western blot. Exposure of HBx-HepG2 cells to hypoxia (5 % O2, 3 % O2, 1 % O2) for 48 h increased the chemo-resistance to 5-fluorouracil (5-FU) (50-1600 µM) and cisplatin (25-800 µM), reduced MMP, and caused the cell cycle arrest at G0/G1 phase in a concentration-dependent manner. Hypoxia also concentration-dependently (5 % O2, 3 % O2, 1 % O2) reduced mRNA expression level of P-glycoprotein (P-gp), multidrug resistance protein (MRP1), lung resistance protein (LRP), and decreased the protein expression level of hypoxia-inducible factor-1α (HIF-1α), P-gp MRP1, and LRP. Following pretreatment with As2O3 at a non-cytotoxic concentration re-sensitized the hypoxia (1 % O2)-induced chemo-resistance to 5-FU and cisplatin in HBx-HepG2 cells. As2O3 pretreatment also prevented MMP reduction and G0/G1 arrest induced by hypoxia. Meanwhile, As2O3 antagonized increase of HIF-1α protein induced by hypoxia, and it also suppresses the increase in expression levels of P-gp, MRP1, and LRP mRNA and proteins. In addition, As2O3 in combination with 5-FU treatment caused up-regulation of DR5, caspase 3, caspase 8, and caspase 9, and down-regulation of BCL-2, but had no effect of DR4.
Conclusions: Our results may suggest that As2O3 re-sensitizes hypoxia-induced chemo-resistance in HBx-HepG2 via complex pathways, and As2O3 may be a potential agent that given in combination with other anti-drugs for the treatment of HBV related HCC, which is resistant to chemotherapy.
Keywords: 5-FU; Chemo-resistance; Cisplatin; HBV; HBx-HepG2; HCC; HIF-1α; LRP; MRP1; P-gp.
Figures






References
-
- Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Yoo BC, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Baltimore: Hepatology; 2015. - PubMed
-
- Yin D, Huang P, Zhuang B, Zhang H, Yan H, Xiao Z, Li W, Zhang J, Tang Q, Hu K, et al. Hepatitis B virus X protein (HBx) is responsible for resistance to targeted therapies in hepatocellular carcinoma: ex vivo culture evidence. Clin Cancer Res. 2015;21(49):4420–4430. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

