Delayed peripheral nerve repair: methods, including surgical 'cross-bridging' to promote nerve regeneration
- PMID: 26692833
- PMCID: PMC4660729
- DOI: 10.4103/1673-5374.167747
Delayed peripheral nerve repair: methods, including surgical 'cross-bridging' to promote nerve regeneration
Erratum in
-
Corrigendum: Delayed peripheral nerve repair: methods, including surgical 'cross-bridging' to promote nerve regeneration.Neural Regen Res. 2021 Feb;16(2):349. doi: 10.4103/1673-5374.290906. Neural Regen Res. 2021. PMID: 32859796 Free PMC article.
Abstract
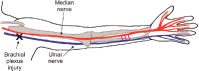
Despite the capacity of Schwann cells to support peripheral nerve regeneration, functional recovery after nerve injuries is frequently poor, especially for proximal injuries that require regenerating axons to grow over long distances to reinnervate distal targets. Nerve transfers, where small fascicles from an adjacent intact nerve are coapted to the nerve stump of a nearby denervated muscle, allow for functional return but at the expense of reduced numbers of innervating nerves. A 1-hour period of 20 Hz electrical nerve stimulation via electrodes proximal to an injury site accelerates axon outgrowth to hasten target reinnervation in rats and humans, even after delayed surgery. A novel strategy of enticing donor axons from an otherwise intact nerve to grow through small nerve grafts (cross-bridges) into a denervated nerve stump, promotes improved axon regeneration after delayed nerve repair. The efficacy of this technique has been demonstrated in a rat model and is now in clinical use in patients undergoing cross-face nerve grafting for facial paralysis. In conclusion, brief electrical stimulation, combined with the surgical technique of promoting the regeneration of some donor axons to 'protect' chronically denervated Schwann cells, improves nerve regeneration and, in turn, functional outcomes in the management of peripheral nerve injuries.
Keywords: Schwann cells; axon regeneration; electrical nerve stimulation; nerve regeneration; nerve repair; peripheral nerve injury.
Figures

References
-
- Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015;269:142–153. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
