Designer Hydrogels for Precision Control of Oxygen Tension and Mechanical Properties
- PMID: 26693017
- PMCID: PMC4675150
- DOI: 10.1039/C5TB01038A
Designer Hydrogels for Precision Control of Oxygen Tension and Mechanical Properties
Abstract
Oxygen levels and mechanical properties provide vital cues to regulate myriad cellular functions and stem cell fate decisions. Here, we present a hybrid hydrogel system in which we can control independently oxygen levels and mechanical properties. We designed, synthesized and analyzed a hybrid hydrogel system comprised of two polymer backbones, gelatin and dextran. Both polymers were crosslinked via a laccase-mediated, oxygen consuming reaction. By specifically controlling the concentration of phenolic molecules available to react in our hydrogel, we could precisely control the time in which the hydrogel remained hypoxic (TH). We were able to achieve a range of TH from the order of minutes to greater than 10 hours. Additionally, by incorporating a secondary crosslinker, transglutaminase, mechanical properties could be adjusted in a user-defined fashion, with dynamic elastic modulus (G') values ranging from <20 Pa to >1 kPa. Importantly, oxygen levels and substrate mechanical properties could be individually tuned and decoupled in our hybrid hydrogels, while retaining the potential to study possible synergistic effects between the two parameters. By precisely controlling oxygen tension and mechanical properties, we expect that research utilizing the new hybrid hydrogels will enhance our understanding of the complex 3D cellular processes mediated by each parameter individually and may also hold clinical interest as acellular therapies.
Figures

 ) and tyramine (TA,
) and tyramine (TA,
 ) conjugated to Gtn and Dex, respectively, forming crosslinked polymer networks. (B) Additional non-conjugated phenol-containing molecules (e.g. TA,
) conjugated to Gtn and Dex, respectively, forming crosslinked polymer networks. (B) Additional non-conjugated phenol-containing molecules (e.g. TA,
 ) are added prior to the reaction, resulting in decreased cross-linking density, and thus slightly decreased stiffness. The same reaction kinetics are employed, resulting in nearly identical oxygen consumption and a decoupling of oxygen concentration and mechanical properties. (C) Secondary crosslinking via transglutaminase-mediated dimerization of primary amines (
) are added prior to the reaction, resulting in decreased cross-linking density, and thus slightly decreased stiffness. The same reaction kinetics are employed, resulting in nearly identical oxygen consumption and a decoupling of oxygen concentration and mechanical properties. (C) Secondary crosslinking via transglutaminase-mediated dimerization of primary amines (
 ) present on both polymer backbones leads to increased stiffness, while not affecting oxygen consumption. By controlling the number of phenolic moieties (both conjugated and unconjugated), as well as controlling the crosslinking density by secondary crosslinking, precise control over both oxygen concentration and mechanical stiffness may be acheived.
) present on both polymer backbones leads to increased stiffness, while not affecting oxygen consumption. By controlling the number of phenolic moieties (both conjugated and unconjugated), as well as controlling the crosslinking density by secondary crosslinking, precise control over both oxygen concentration and mechanical stiffness may be acheived.

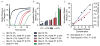

 ). Scale bars 100 μm.
). Scale bars 100 μm.Similar articles
-
O2-controllable hydrogels for studying cellular responses to hypoxic gradients in three dimensions in vitro and in vivo.Nat Protoc. 2017 Aug;12(8):1620-1638. doi: 10.1038/nprot.2017.059. Epub 2017 Jul 20. Nat Protoc. 2017. PMID: 28726849 Free PMC article.
-
Enzymatically cross-linked gelatin-phenol hydrogels with a broader stiffness range for osteogenic differentiation of human mesenchymal stem cells.Acta Biomater. 2012 May;8(5):1826-37. doi: 10.1016/j.actbio.2012.02.002. Epub 2012 Feb 8. Acta Biomater. 2012. PMID: 22343003
-
Thermal behavior and mechanical properties of physically crosslinked PVA/Gelatin hydrogels.J Mech Behav Biomed Mater. 2010 Feb;3(2):203-9. doi: 10.1016/j.jmbbm.2009.07.001. Epub 2009 Jul 14. J Mech Behav Biomed Mater. 2010. PMID: 20129419
-
Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness.Tissue Eng Part A. 2014 Aug;20(15-16):2131-9. doi: 10.1089/ten.tea.2013.0531. Epub 2014 Apr 7. Tissue Eng Part A. 2014. PMID: 24707837
-
Mechanical properties and in vitro behavior of nanofiber-hydrogel composites for tissue engineering applications.Nanotechnology. 2012 Mar 9;23(9):095705. doi: 10.1088/0957-4484/23/9/095705. Epub 2012 Feb 10. Nanotechnology. 2012. PMID: 22322583
Cited by
-
Stabilization of enzyme-immobilized hydrogels for extended hypoxic cell culture.Emergent Mater. 2019 Jun;2(2):263-272. doi: 10.1007/s42247-019-00038-4. Epub 2019 Jul 17. Emergent Mater. 2019. PMID: 37502125 Free PMC article.
-
Discretizing Three-Dimensional Oxygen Gradients to Modulate and Investigate Cellular Processes.Adv Sci (Weinh). 2021 Jul;8(14):e2100190. doi: 10.1002/advs.202100190. Epub 2021 Jun 21. Adv Sci (Weinh). 2021. PMID: 34151527 Free PMC article.
-
Gradient Hydrogels.Adv Biochem Eng Biotechnol. 2021;178:227-251. doi: 10.1007/10_2020_155. Adv Biochem Eng Biotechnol. 2021. PMID: 33219386
-
Dual IFN-γ/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms.J Immunol Regen Med. 2018 Mar;1:45-56. doi: 10.1016/j.regen.2018.01.001. Epub 2018 Apr 25. J Immunol Regen Med. 2018. PMID: 30364570 Free PMC article.
-
Tissue Engineering Approaches to Uncover Therapeutic Targets for Endothelial Dysfunction in Pathological Microenvironments.Int J Mol Sci. 2022 Jul 3;23(13):7416. doi: 10.3390/ijms23137416. Int J Mol Sci. 2022. PMID: 35806421 Free PMC article. Review.
References
-
- Park KM, et al. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomaterialia. 2009;5(6):1956–1965. - PubMed
-
- Park JS, et al. In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application. Journal of Controlled Release. 2007;124(1–2):51–59. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

