Sustained Small Molecule Delivery from Injectable Hyaluronic Acid Hydrogels through Host-Guest Mediated Retention
- PMID: 26693019
- PMCID: PMC4675358
- DOI: 10.1039/C5TB00981B
Sustained Small Molecule Delivery from Injectable Hyaluronic Acid Hydrogels through Host-Guest Mediated Retention
Abstract
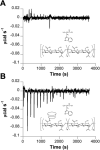
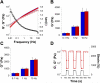
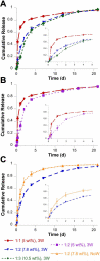
Self-assembled and injectable hydrogels have many beneficial properties for the local delivery of therapeutics; however, challenges still exist in the sustained release of small molecules from these highly hydrated networks. Host-guest chemistry between cyclodextrin and adamantane has been used to create supramolecular hydrogels from modified polymers. Beyond assembly, this chemistry may also provide increased drug retention and sustained release through the formation of inclusion complexes between drugs and cyclodextrin. Here, we engineered a two-component system from adamantane-modified and β-cyclodextrin (CD)-modified hyaluronic acid (HA), a natural component of the extracellular matrix, to produce hydrogels that are both injectable and able to sustain the release of small molecules. The conjugation of cyclodextrin to HA dramatically altered its affinity for hydrophobic small molecules, such as tryptophan. This interaction led to lower molecule diffusivity and the release of small molecules for up to 21 days with release profiles dependent on CD concentration and drug-CD affinity. There was significant attenuation of release from the supramolecular hydrogels (~20% release in 24h) when compared to hydrogels without CD (~90% release in 24h). The loading of small molecules also had no effect on hydrogel mechanics or self-assembly properties. Finally, to illustrate this controlled delivery approach with clinically used small molecule pharmaceuticals, we sustained the release of two widely used drugs (i.e., doxycycline and doxorubicin) from these hydrogels.
Keywords: cyclodextrin; drug delivery; host-guest chemistry; hydrogel; self-assembly; small molecules; sustained release.
Figures

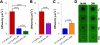
Similar articles
-
A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release.Acta Biomater. 2021 Sep 1;131:286-301. doi: 10.1016/j.actbio.2021.07.004. Epub 2021 Jul 8. Acta Biomater. 2021. PMID: 34246803
-
Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels.Biomacromolecules. 2013 Nov 11;14(11):4125-34. doi: 10.1021/bm401280z. Epub 2013 Oct 14. Biomacromolecules. 2013. PMID: 24070551 Free PMC article.
-
Guest-Host Supramolecular Assembly of Injectable Hydrogel Nanofibers for Cell Encapsulation.ACS Biomater Sci Eng. 2021 Sep 13;7(9):4164-4174. doi: 10.1021/acsbiomaterials.1c00275. Epub 2021 Apr 23. ACS Biomater Sci Eng. 2021. PMID: 33891397
-
Cyclodextrin-Mediated Hierarchical Self-Assembly and Its Potential in Drug Delivery Applications.J Pharm Sci. 2016 Sep;105(9):2570-2588. doi: 10.1016/j.xphs.2016.05.010. Epub 2016 Jun 22. J Pharm Sci. 2016. PMID: 27342436 Review.
-
[Functionalization of Cyclodextrin Derivatives to Create Supramolecular Pharmaceutical Materials].Yakugaku Zasshi. 2019;139(2):165-173. doi: 10.1248/yakushi.18-00168-3. Yakugaku Zasshi. 2019. PMID: 30713225 Review. Japanese.
Cited by
-
Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery.Gels. 2020 May 8;6(2):14. doi: 10.3390/gels6020014. Gels. 2020. PMID: 32397180 Free PMC article. Review.
-
Injectable Granular Hydrogels Enable Avidity-Controlled Biotherapeutic Delivery.ACS Biomater Sci Eng. 2024 Mar 11;10(3):1577-1588. doi: 10.1021/acsbiomaterials.3c01906. Epub 2024 Feb 15. ACS Biomater Sci Eng. 2024. PMID: 38357739 Free PMC article.
-
Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects.ACS Cent Sci. 2019 Mar 27;5(3):440-450. doi: 10.1021/acscentsci.8b00764. Epub 2019 Feb 13. ACS Cent Sci. 2019. PMID: 30937371 Free PMC article.
-
Supramolecular Prodrug Nanovectors for Active Tumor Targeting and Combination Immunotherapy of Colorectal Cancer.Adv Sci (Weinh). 2020 Feb 25;7(8):1903332. doi: 10.1002/advs.201903332. eCollection 2020 Apr. Adv Sci (Weinh). 2020. PMID: 32328426 Free PMC article.
-
Engineered Axonal Tracts as "Living Electrodes" for Synaptic-Based Modulation of Neural Circuitry.Adv Funct Mater. 2018 Mar 21;28(12):1701183. doi: 10.1002/adfm.201701183. Epub 2017 Sep 4. Adv Funct Mater. 2018. PMID: 34045935 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

