The Impact of Paeoniflorin on α-Synuclein Degradation Pathway
- PMID: 26693241
- PMCID: PMC4674600
- DOI: 10.1155/2015/182495
The Impact of Paeoniflorin on α-Synuclein Degradation Pathway
Abstract
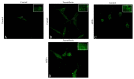
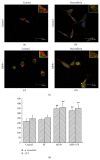
Paeoniflorin (PF) is the major active ingredient in the traditional Chinese medicine Radix. It plays a neuroprotective role by regulating autophagy and the ubiquitin-proteasome degradation pathway. In this study, we found PF significantly reduced cell damage caused by MPP+, returning cells to normal state. Cell viability significantly improved after 24 h exposure to RAPA and PF in the MPP+ group (all P < 0.01). CAT and SOD activities were significantly decreased after PF and RAPA treatment, compared with MPP+ (P < 0.001). In addition, MPP+ activated both LC3-II and E1; RAPA increased LC3-II but inhibited E1. PF significantly upregulated both LC3-II (autophagy) and E1 (ubiquitin-proteasome pathway) expression (P < 0.001), promoted degradation of α-synuclein, and reduced cell damage. We show MPP+ enhanced immunofluorescence signal of intracellular α-synuclein and LC3. Fluorescence intensity of α-synuclein decreased after PF treatment. In conclusion, these data show PF reversed the decline of proteasome activity caused by MPP+ and significantly upregulated both autophagy and ubiquitin-proteasome pathways, promoted the degradation of α-synuclein, and reduced cell damage. These findings suggest PF is a potential therapeutic medicine for neurodegenerative diseases.
Figures





References
-
- Lin B. Polyphenols and neuroprotection against ischemia and neurodegeneration. Mini-Reviews in Medicinal Chemistry. 2011;11(14):1222–1238. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

