Oxidative Stress in Placenta: Health and Diseases
- PMID: 26693479
- PMCID: PMC4676991
- DOI: 10.1155/2015/293271
Oxidative Stress in Placenta: Health and Diseases
Abstract
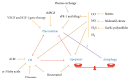
During pregnancy, development of the placenta is interrelated with the oxygen concentration. Embryo development takes place in a low oxygen environment until the beginning of the second trimester when large amounts of oxygen are conveyed to meet the growth requirements. High metabolism and oxidative stress are common in the placenta. Reactive oxidative species sometimes harm placental development, but they are also reported to regulate gene transcription and downstream activities such as trophoblast proliferation, invasion, and angiogenesis. Autophagy and apoptosis are two crucial, interconnected processes in the placenta that are often influenced by oxidative stress. The proper interactions between them play an important role in placental homeostasis. However, an imbalance between the protective and destructive mechanisms of autophagy and apoptosis seems to be linked with pregnancy-related disorders such as miscarriage, preeclampsia, and intrauterine growth restriction. Thus, potential therapies to hold oxidative stress in leash, promote placentation, and avoid unwanted apoptosis are discussed.
Figures


Similar articles
-
Placental oxidative stress: from miscarriage to preeclampsia.J Soc Gynecol Investig. 2004 Sep;11(6):342-52. doi: 10.1016/j.jsgi.2004.03.003. J Soc Gynecol Investig. 2004. PMID: 15350246 Review.
-
Oxidative Stress: Placenta Function and Dysfunction.Am J Reprod Immunol. 2016 Oct;76(4):258-71. doi: 10.1111/aji.12454. Epub 2015 Nov 21. Am J Reprod Immunol. 2016. PMID: 26589876 Review.
-
Angiogenesis in the placenta: the role of reactive oxygen species signaling.Biomed Res Int. 2015;2015:814543. doi: 10.1155/2015/814543. Epub 2015 Jan 29. Biomed Res Int. 2015. PMID: 25705690 Free PMC article. Review.
-
Oxidative Stress in Preeclampsia and Placental Diseases.Int J Mol Sci. 2018 May 17;19(5):1496. doi: 10.3390/ijms19051496. Int J Mol Sci. 2018. PMID: 29772777 Free PMC article. Review.
-
Involvement of oxidative stress in placental dysfunction, the pathophysiology of fetal death and pregnancy disorders.Reproduction. 2023 Jul 5;166(2):R25-R38. doi: 10.1530/REP-22-0278. Print 2023 Aug 1. Reproduction. 2023. PMID: 37318094 Review.
Cited by
-
Anti-Apoptotic Effect of Apelin in Human Placenta: Studies on BeWo Cells and Villous Explants from Third-Trimester Human Pregnancy.Int J Mol Sci. 2021 Mar 9;22(5):2760. doi: 10.3390/ijms22052760. Int J Mol Sci. 2021. PMID: 33803239 Free PMC article.
-
AMP-activated protein kinase activator AICAR attenuates hypoxia-induced murine fetal growth restriction in part by improving uterine artery blood flow.J Physiol. 2020 Sep;598(18):4093-4105. doi: 10.1113/JP279341. Epub 2020 Jul 6. J Physiol. 2020. PMID: 32592403 Free PMC article.
-
Antioxidants and Oxidative Stress: Focus in Obese Pregnancies.Front Physiol. 2018 Nov 6;9:1569. doi: 10.3389/fphys.2018.01569. eCollection 2018. Front Physiol. 2018. PMID: 30459642 Free PMC article.
-
The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside.Oxid Med Cell Longev. 2018 Jul 24;2018:7483062. doi: 10.1155/2018/7483062. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30140369 Free PMC article. Review.
-
Epitranscriptomic profiling in human placenta: N6-methyladenosine modification at the 5'-untranslated region is related to fetal growth and preeclampsia.FASEB J. 2020 Jan;34(1):494-512. doi: 10.1096/fj.201900619RR. Epub 2019 Nov 25. FASEB J. 2020. PMID: 31914637 Free PMC article.
References
-
- Ďuračková Z. Some current insights into oxidative stress. Physiological Research. 2010;59(4):459–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

