The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice
- PMID: 26693700
- PMCID: PMC4915993
- DOI: 10.1038/mi.2015.122
The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice
Abstract
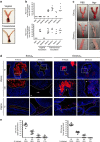
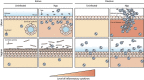
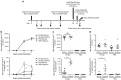
Women with asymptomatic Neisseria gonorrhoeae infection are at risk of developing pelvic inflammatory disease (PID) if the bacteria ascend from the endocervix into the uterus and oviducts. Factors that affect disease severity, ranging from mild discomfort to severe inflammation, pain, and infertility, remain elusive. Herein we perform direct transcervical inoculation of N. gonorrhoeae into the uterus of mice to establish an infection that leads to PID. Profoundly different disease outcomes were apparent at different stages of the reproductive cycle. Mice that were infected during the diestrus stage of the reproductive cycle displayed extensive gonococcal penetration into the submucosa, severe inflammation, and clinical signs reflecting discomfort. Meanwhile, infection during the intervening estrus stage showed only modest effects. Furthermore, a gonococcal-specific humoral response was only elicited following the penetrative upper genital tract (UGT) infection during diestrus but not estrus. Strikingly, the potential for antibodies to contribute to protection during re-infection also depends upon the reproductive stage, as antigonococcal antibodies within the genital tract were markedly higher when mice were in diestrus. Combined, this work establishes a robust new model reflecting gonococcal PID in humans and reveals how the reproductive cycle determines the pathogenic outcome of gonococcal infections of the UGT.
Figures





References
-
- Wira, C.R., Fahey, J.V., Sentman, C.L., Pioli, P.A. & Shen, L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206, 306–335 (2005). - PubMed
-
- Givan, A.L. et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 38, 350–359 (1997). - PubMed
-
- Pudney, J., Quayle, A.J. & Anderson, D.J. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 73, 1253–1263 (2005). - PubMed
-
- Hook, E.W. 3rd & Holmes, K.K. Gonococcal infections. Ann. Intern. Med. 102, 229–243 (1985). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

