Physician response to implementation of genotype-tailored antiplatelet therapy
- PMID: 26693963
- PMCID: PMC4899238
- DOI: 10.1002/cpt.331
Physician response to implementation of genotype-tailored antiplatelet therapy
Abstract
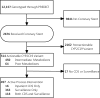
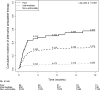
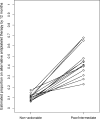
Physician responses to genomic information are vital to the success of precision medicine initiatives. We prospectively studied a pharmacogenomics implementation program for the propensity of clinicians to select antiplatelet therapy based on CYP2C19 loss-of-function variants in stented patients. Among 2,676 patients, 514 (19.2%) were found to have a CYP2C19 variant affecting clopidogrel metabolism. For the majority (93.6%) of the cohort, cardiologists received active and direct notification of CYP2C19 status. Over 12 months, 57.6% of poor metabolizers and 33.2% of intermediate metabolizers received alternatives to clopidogrel. CYP2C19 variant status was the most influential factor impacting the prescribing decision (hazard ratio [HR] in poor metabolizers 8.1, 95% confidence interval [CI] [5.4, 12.2] and HR 5.0, 95% CI [4.0, 6.3] in intermediate metabolizers), followed by patient age and type of stent implanted. We conclude that cardiologists tailored antiplatelet therapy for a minority of patients with a CYP2C19 variant and considered both genomic and nongenomic risks in their clinical decision-making.
© 2015 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Figures

Similar articles
-
Impact of CYP2C19 genetic testing on provider prescribing patterns for antiplatelet therapy after acute coronary syndromes and percutaneous coronary intervention.Circ Cardiovasc Qual Outcomes. 2013 Nov;6(6):694-9. doi: 10.1161/CIRCOUTCOMES.113.000321. Epub 2013 Nov 5. Circ Cardiovasc Qual Outcomes. 2013. PMID: 24192573
-
Effect of CYP2C19 Polymorphisms on the Platelet Response to Clopidogrel and Influence on the Effect of High Versus Standard Dose Clopidogrel in Carotid Artery Stenting.Eur J Vasc Endovasc Surg. 2016 Feb;51(2):175-86. doi: 10.1016/j.ejvs.2015.09.020. Epub 2015 Oct 31. Eur J Vasc Endovasc Surg. 2016. PMID: 26526111
-
[Impact of CYP2C19 genotype and platelet function on clinical outcome in coronary atherosclerotic heart diseases patients received clopidogrel post percutaneous coronary intervention].Zhonghua Xin Xue Guan Bing Za Zhi. 2017 May 24;45(5):377-385. doi: 10.3760/cma.j.issn.0253-3758.2017.05.004. Zhonghua Xin Xue Guan Bing Za Zhi. 2017. PMID: 28511321 Chinese.
-
Role of CYP2C19 genotype testing in clinical use of clopidogrel: is it really useful?Expert Rev Cardiovasc Ther. 2018 May;16(5):369-377. doi: 10.1080/14779072.2018.1459186. Epub 2018 Apr 3. Expert Rev Cardiovasc Ther. 2018. PMID: 29589775 Review.
-
Personalizing antiplatelet prescribing using genetics for patients undergoing percutaneous coronary intervention.Expert Rev Cardiovasc Ther. 2017 Aug;15(8):581-589. doi: 10.1080/14779072.2017.1355236. Epub 2017 Jul 19. Expert Rev Cardiovasc Ther. 2017. PMID: 28699807 Free PMC article. Review.
Cited by
-
Cost-effectiveness analysis of CYP3A5 genotype-guided tacrolimus dosing in solid organ transplantation using real-world data.Pharmacogenomics J. 2024 May 15;24(3):14. doi: 10.1038/s41397-024-00334-1. Pharmacogenomics J. 2024. PMID: 38750044
-
A Decision-Theoretic Approach to Panel-Based, Preemptive Genotyping.MDM Policy Pract. 2019 Aug 17;4(2):2381468319864337. doi: 10.1177/2381468319864337. eCollection 2019 Jul-Dec. MDM Policy Pract. 2019. PMID: 31453360 Free PMC article.
-
Personalised antiplatelet therapy based on pharmacogenomics in acute ischaemic minor stroke and transient ischaemic attack: study protocol for a randomised controlled trial.BMJ Open. 2019 May 22;9(5):e028595. doi: 10.1136/bmjopen-2018-028595. BMJ Open. 2019. PMID: 31123001 Free PMC article.
-
Genetic Determinants of P2Y12 Inhibitors and Clinical Implications.Interv Cardiol Clin. 2017 Jan;6(1):141-149. doi: 10.1016/j.iccl.2016.08.010. Interv Cardiol Clin. 2017. PMID: 27886818 Free PMC article. Review.
-
Pharmacogenomics-Based Point-of-Care Clinical Decision Support Significantly Alters Drug Prescribing.Clin Pharmacol Ther. 2017 Nov;102(5):859-869. doi: 10.1002/cpt.709. Epub 2017 Jun 15. Clin Pharmacol Ther. 2017. PMID: 28398598 Free PMC article.
References
-
- Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief. 2010:1–8. - PubMed
-
- Pharmacogenomic Biomarkers in Drug Labels. FDA Pharmacogenomic Biomarkers in Drug Labeling. at < http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/...>.
-
- Stanek EJ, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther. 2012;91:450–458. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

