Weaver Syndrome-Associated EZH2 Protein Variants Show Impaired Histone Methyltransferase Function In Vitro
- PMID: 26694085
- PMCID: PMC4832389
- DOI: 10.1002/humu.22946
Weaver Syndrome-Associated EZH2 Protein Variants Show Impaired Histone Methyltransferase Function In Vitro
Abstract
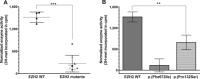
Weaver syndrome (WS) is a rare congenital disorder characterized by generalized overgrowth, macrocephaly, specific facial features, accelerated bone age, intellectual disability, and susceptibility to cancers. De novo mutations in the enhancer of zeste homolog 2 (EZH2) have been shown to cause WS. EZH2 is a histone methyltransferase that acts as the catalytic agent of the polycomb-repressive complex 2 (PRC2) to maintain gene repression via methylation of lysine 27 on histone H3 (H3K27). Functional studies investigating histone methyltransferase activity of mutant EZH2 from various cancers have been reported, whereas WS-associated mutations remain poorly characterized. To investigate the role of EZH2 in WS, we performed functional studies using artificially assembled PRC2 complexes containing mutagenized human EZH2 that reflected the codon changes predicted from patients with WS. We found that WS-associated amino acid alterations reduce the histone methyltransferase function of EZH2 in this in vitro assay. Our results support the hypothesis that WS is caused by constitutional mutations in EZH2 that alter the histone methyltransferase function of PRC2. However, histone methyltransferase activities of different EZH2 variants do not appear to correlate directly with the phenotypic variability between WS patients and individuals with a common c.553G>C (p.Asp185His) polymorphism in EZH2.
Keywords: EZH2; H3K27; Weaver syndrome; childhood cancer; histone methyltransferase.
© 2015 The Authors. **Human Mutation published by Wiley Periodicals, Inc.
Figures
References
-
- Al‐Salem A, Alshammari MJ, Hassan H, Alazami AM, Alkuraya FS. 2013. Weaver syndrome and defective cortical development: a rare association. Am J Med Genet A 161A:225–227. - PubMed
-
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. 2005. Akt‐mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 310:306–310. - PubMed
-
- Coulter‐Mackie MB, Gagnier L. 2003. Spectrum of mutations in the arylsulfatase A gene in a Canadian DNA collection including two novel frameshift mutations, a new missense mutation (C488R) and an MLD mutation (R84Q) in cis with a pseudodeficiency allele. Mol Genet Metab 79:91–98. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

