Potassium Hexacyanoferrate (III)-Catalyzed Dimerization of Hydroxystilbene: Biomimetic Synthesis of Indane Stilbene Dimers
- PMID: 26694345
- PMCID: PMC6332065
- DOI: 10.3390/molecules201219872
Potassium Hexacyanoferrate (III)-Catalyzed Dimerization of Hydroxystilbene: Biomimetic Synthesis of Indane Stilbene Dimers
Abstract
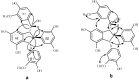
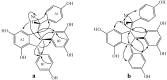
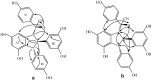
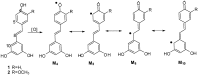
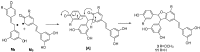
Using potassium hexacyanoferrate (III)-sodium acetate as oxidant, the oxidative coupling reaction of isorhapontigenin and resveratrol in aqueous acetone resulted in the isolation of three new indane dimers 4, 6, and 7, together with six known stilbene dimers. Indane dimer 5 was obtained for the first time by direct transformation from isorhapontigenin. The structures and relative configurations of the dimers were elucidated using spectral analysis, and their possible formation mechanisms were discussed. The results indicate that this reaction could be used as a convenient method for the semi-synthesis of indane dimers because of the mild conditions and simple reaction products.
Keywords: biomimetic synthesis; hydroxystilbene; indane stilbene dimer; potassium hexacyanoferrate (III).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Biomimetic Synthesis of Resveratrol Trimers Catalyzed by Horseradish Peroxidase.Molecules. 2017 May 17;22(5):819. doi: 10.3390/molecules22050819. Molecules. 2017. PMID: 28513542 Free PMC article.
-
Biomimetic synthesis of active isorhapontigenin dimers.J Asian Nat Prod Res. 2014;16(5):511-21. doi: 10.1080/10286020.2014.913030. Epub 2014 May 2. J Asian Nat Prod Res. 2014. PMID: 24786449
-
A scalable biomimetic synthesis of resveratrol dimers and systematic evaluation of their antioxidant activities.Angew Chem Int Ed Engl. 2015 Mar 16;54(12):3754-7. doi: 10.1002/anie.201409773. Epub 2015 Feb 4. Angew Chem Int Ed Engl. 2015. PMID: 25650836 Free PMC article.
-
Stiff-Stilbene Photoswitches: From Fundamental Studies to Emergent Applications.Angew Chem Int Ed Engl. 2020 Aug 3;59(32):13192-13202. doi: 10.1002/anie.202001031. Epub 2020 Jun 2. Angew Chem Int Ed Engl. 2020. PMID: 32222016 Free PMC article. Review.
-
Production of high molecular-ordered stilbene oligomers for the study of their biological activity: total synthesis, bio-catalyzed synthesis and production by plant systems.Nat Prod Rep. 2023 May 24;40(5):1045-1057. doi: 10.1039/d2np00073c. Nat Prod Rep. 2023. PMID: 36880302 Review.
Cited by
-
Oxidative dehydrogenative couplings of alkenyl phenols.Org Biomol Chem. 2021 Oct 6;19(38):8205-8226. doi: 10.1039/d1ob01040a. Org Biomol Chem. 2021. PMID: 34522924 Free PMC article. Review.
-
Biomimetic Synthesis of Resveratrol Trimers Catalyzed by Horseradish Peroxidase.Molecules. 2017 May 17;22(5):819. doi: 10.3390/molecules22050819. Molecules. 2017. PMID: 28513542 Free PMC article.
References
-
- Yao C.S., Wang X.F. Naturally Active Oligostilbenes. J. Asian Nat. Prod. Res. 2016 in press. - PubMed
-
- Lin M., Yao C.S. Natural oligostilbenes. Stud. Nat. Prod. Chem. 2006;33:601–644.
-
- Li W.L., Li H.F., Luo Y.L., Yang Y.H., Wang N. Biosynthesis of resveratrol dimers by regioselective oxidative coupling reaction. Synlett. 2010;8:1247–1250. doi: 10.1055/s-0029-1219787. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

