Unfolded Protein Response and Macroautophagy in Alzheimer's, Parkinson's and Prion Diseases
- PMID: 26694349
- PMCID: PMC6332363
- DOI: 10.3390/molecules201219865
Unfolded Protein Response and Macroautophagy in Alzheimer's, Parkinson's and Prion Diseases
Abstract
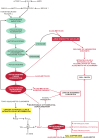
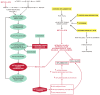
Proteostasis are integrated biological pathways within cells that control synthesis, folding, trafficking and degradation of proteins. The absence of cell division makes brain proteostasis susceptible to age-related changes and neurodegeneration. Two key processes involved in sustaining normal brain proteostasis are the unfolded protein response and autophagy. Alzheimer's disease (AD), Parkinson's disease (PD) and prion diseases (PrDs) have different clinical manifestations of neurodegeneration, however, all share an accumulation of misfolded pathological proteins associated with perturbations in unfolded protein response and macroautophagy. While both the unfolded protein response and macroautophagy play an important role in the prevention and attenuation of AD and PD progression, only macroautophagy seems to play an important role in the development of PrDs. Macroautophagy and unfolded protein response can be modulated by pharmacological interventions. However, further research is necessary to better understand the regulatory pathways of both processes in health and neurodegeneration to be able to develop new therapeutic interventions.
Keywords: Alzheimer’s disease; Parkinson’s disease; autophagy; neurodegeneration; prion diseases; proteostasis; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lipinski M.M., Zheng B., Lu T., Yan Z., Py B.F., Ng A., Xavier R.J., Li C., Yankner B.A., Scherzer C.R., et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2010;107:14164–14169. doi: 10.1073/pnas.1009485107. - DOI - PMC - PubMed
-
- Heiseke A., Aguib Y., Schatzl H.M. Autophagy, prion infection and their mutual interactions. Curr. Issues Mol. Biol. 2010;12:87–97. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

