A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips
- PMID: 26694413
- PMCID: PMC4721812
- DOI: 10.3390/s151229893
A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips
Abstract
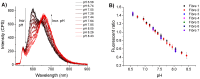
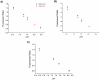
This paper demonstrates the first single optical fibre tip probe for concurrent detection of both hydrogen peroxide (H₂O₂) concentration and pH of a solution. The sensor is constructed by embedding two fluorophores: carboxyperoxyfluor-1 (CPF1) and seminaphtharhodafluor-2 (SNARF2) within a polymer matrix located on the tip of the optical fibre. The functionalised fibre probe reproducibly measures pH, and is able to accurately detect H₂O₂ over a biologically relevant concentration range. This sensor offers potential for non-invasive detection of pH and H₂O₂ in biological environments using a single optical fibre.
Keywords: dual sensor; fibre tip sensor; hydrogen peroxide probe; optical fibre; pH sensor.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

