MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt's Lymphoma Cells to Trigger Apoptosis through Multiple Pathways
- PMID: 26694420
- PMCID: PMC4699244
- DOI: 10.3390/md13127071
MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt's Lymphoma Cells to Trigger Apoptosis through Multiple Pathways
Abstract
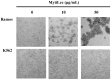
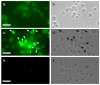
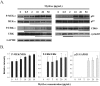
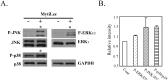
MytiLec; a novel lectin isolated from the Mediterranean mussel (Mytilus galloprovincialis); shows strong binding affinity to globotriose (Gb3: Galα1-4Galβ1-4Glc). MytiLec revealed β-trefoil folding as also found in the ricin B-subunit type (R-type) lectin family, although the amino acid sequences were quite different. Classification of R-type lectin family members therefore needs to be based on conformation as well as on primary structure. MytiLec specifically killed Burkitt's lymphoma Ramos cells, which express Gb3. Fluorescein-labeling assay revealed that MytiLec was incorporated inside the cells. MytiLec treatment of Ramos cells resulted in activation of both classical MAPK/ extracellular signal-regulated kinase and extracellular signal-regulated kinase (MEK-ERK) and stress-activated (p38 kinase and JNK) Mitogen-activated protein kinases (MAPK) pathways. In the cells, MytiLec treatment triggered expression of tumor necrosis factor (TNF)-α (a ligand of death receptor-dependent apoptosis) and activation of mitochondria-controlling caspase-9 (initiator caspase) and caspase-3 (activator caspase). Experiments using the specific MEK inhibitor U0126 showed that MytiLec-induced phosphorylation of the MEK-ERK pathway up-regulated expression of the cyclin-dependent kinase inhibitor p21, leading to cell cycle arrest and TNF-α production. Activation of caspase-3 by MytiLec appeared to be regulated by multiple different pathways. Our findings, taken together, indicate that the novel R-type lectin MytiLec initiates programmed cell death of Burkitt's lymphoma cells through multiple pathways (MAPK cascade, death receptor signaling; caspase activation) based on interaction of the lectin with Gb3-containing glycosphingolipid-enriched microdomains on the cell surface.
Keywords: Burkitt’s lymphoma cells; JNK; MEK/ERK; MytiLec; Mytilus galloprovincialis; R-type lectin; TNF-α; caspase-9/3; globotriose (Gb3); p21: p38 kinase; β-trefoil.
Figures



Similar articles
-
Purification and Functional Characterization of the Effects on Cell Signaling of Mytilectin: A Novel β-Trefoil Lectin from Marine Mussels.Methods Mol Biol. 2020;2132:201-213. doi: 10.1007/978-1-0716-0430-4_21. Methods Mol Biol. 2020. PMID: 32306329
-
A lectin from the mussel Mytilus galloprovincialis has a highly novel primary structure and induces glycan-mediated cytotoxicity of globotriaosylceramide-expressing lymphoma cells.J Biol Chem. 2012 Dec 28;287(53):44772-83. doi: 10.1074/jbc.M112.418012. Epub 2012 Oct 23. J Biol Chem. 2012. PMID: 23093409 Free PMC article.
-
[Cell Function Research of β-Trefoil Lectins from Mytilidae].Yakugaku Zasshi. 2021;141(4):481-488. doi: 10.1248/yakushi.20-00215. Yakugaku Zasshi. 2021. PMID: 33790114 Review. Japanese.
-
Catfish rhamnose-binding lectin induces G0/1 cell cycle arrest in Burkitt's lymphoma cells via membrane surface Gb3.Glycoconj J. 2017 Feb;34(1):127-138. doi: 10.1007/s10719-016-9739-2. Epub 2016 Oct 28. Glycoconj J. 2017. PMID: 27796613
-
[Application of Lectin from Catfish Eggs to Cancer Therapy: A Fundamental Study].Yakugaku Zasshi. 2018;138(12):1451-1459. doi: 10.1248/yakushi.18-00128. Yakugaku Zasshi. 2018. PMID: 30504657 Review. Japanese.
Cited by
-
Differential expression of N-linked oligosaccharides in methotrexate-resistant primary central nervous system lymphoma cells.BMC Cancer. 2019 Sep 11;19(1):910. doi: 10.1186/s12885-019-6129-8. BMC Cancer. 2019. PMID: 31510952 Free PMC article.
-
The choanoflagellate pore-forming lectin SaroL-1 punches holes in cancer cells by targeting the tumor-related glycosphingolipid Gb3.Commun Biol. 2022 Sep 12;5(1):954. doi: 10.1038/s42003-022-03869-w. Commun Biol. 2022. PMID: 36097056 Free PMC article.
-
cDNA and Gene Structure of MytiLec-1, A Bacteriostatic R-Type Lectin from the Mediterranean Mussel (Mytilus galloprovincialis).Mar Drugs. 2016 May 11;14(5):92. doi: 10.3390/md14050092. Mar Drugs. 2016. PMID: 27187419 Free PMC article.
-
A Shiga Toxin B-Subunit-Based Lectibody Boosts T Cell Cytotoxicity towards Gb3-Positive Cancer Cells.Cells. 2023 Jul 20;12(14):1896. doi: 10.3390/cells12141896. Cells. 2023. PMID: 37508560 Free PMC article.
-
Ulva pertusa lectin 1 delivery through adenovirus vector affects multiple signaling pathways in cancer cells.Glycoconj J. 2017 Aug;34(4):489-498. doi: 10.1007/s10719-017-9767-6. Epub 2017 Mar 27. Glycoconj J. 2017. PMID: 28349379
References
-
- Gerdol M., Venier P. An updated molecular basis for mussel immunity. Fish Shellfish Immunol. 2015;46:17–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

