Structure-Activity Relationship and in Vivo Anti-Tumor Evaluations of Dictyoceratin-A and -C, Hypoxia-Selective Growth Inhibitors from Marine Sponge
- PMID: 26694423
- PMCID: PMC4699247
- DOI: 10.3390/md13127074
Structure-Activity Relationship and in Vivo Anti-Tumor Evaluations of Dictyoceratin-A and -C, Hypoxia-Selective Growth Inhibitors from Marine Sponge
Abstract
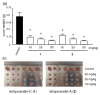
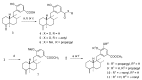
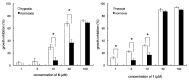
Oral dictyoceratin-C (1) and A (2), hypoxia-selective growth inhibitors, showed potent in vivo antitumor effects in mice subcutaneously inoculated with sarcoma S180 cells. Structurally modified analogs were synthesized to assess the structure-activity relationship of the natural compounds 1 and 2 isolated from a marine sponge. Biological evaluation of these analogs showed that the exo-olefin and hydroxyl and methyl ester moieties were important for the hypoxia-selective growth inhibitory activities of 1 and 2. Thus far, only substitution of the methyl ester with propargyl amide in 1 was found to be effective for the synthesis of probe molecules for target identification.
Keywords: analog synthesis; dictyoceratin-A; dictyoceratin-C; hypoxia-selective growth inhibitor; in vivo antitumor effect; marine sponge; structure-activity relationship.
Figures





Similar articles
-
Target Identification of the Marine Natural Products Dictyoceratin-A and -C as Selective Growth Inhibitors in Cancer Cells Adapted to Hypoxic Environments.Mar Drugs. 2019 Mar 8;17(3):163. doi: 10.3390/md17030163. Mar Drugs. 2019. PMID: 30857246 Free PMC article.
-
Marine spongian sesquiterpene phenols, dictyoceratin-C and smenospondiol, display hypoxia-selective growth inhibition against cancer cells.Bioorg Med Chem Lett. 2014 Jul 15;24(14):3155-7. doi: 10.1016/j.bmcl.2014.04.116. Epub 2014 May 9. Bioorg Med Chem Lett. 2014. PMID: 24865416
-
Enantioselective synthesis of dictyoceratin-A (smenospondiol) and -C, hypoxia-selective growth inhibitors from marine sponge.Bioorg Med Chem. 2015 Mar 1;23(5):966-75. doi: 10.1016/j.bmc.2015.01.021. Epub 2015 Jan 19. Bioorg Med Chem. 2015. PMID: 25659617
-
Sesquiterpenes: natural products that decrease cancer growth.Curr Med Chem Anticancer Agents. 2005 Sep;5(5):477-99. doi: 10.2174/1568011054866973. Curr Med Chem Anticancer Agents. 2005. PMID: 16178774 Review.
-
[Creation of readily accessible analogue of cortistatin A as an antitumor drug lead].Yakugaku Zasshi. 2013;133(8):867-72. doi: 10.1248/yakushi.13-00159. Yakugaku Zasshi. 2013. PMID: 23903226 Review. Japanese.
Cited by
-
Dactylospongia elegans-A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship.Mar Drugs. 2022 Mar 23;20(4):221. doi: 10.3390/md20040221. Mar Drugs. 2022. PMID: 35447894 Free PMC article. Review.
-
Target Identification of the Marine Natural Products Dictyoceratin-A and -C as Selective Growth Inhibitors in Cancer Cells Adapted to Hypoxic Environments.Mar Drugs. 2019 Mar 8;17(3):163. doi: 10.3390/md17030163. Mar Drugs. 2019. PMID: 30857246 Free PMC article.
-
From Seabed to Bedside: A Review on Promising Marine Anticancer Compounds.Biomolecules. 2020 Feb 6;10(2):248. doi: 10.3390/biom10020248. Biomolecules. 2020. PMID: 32041255 Free PMC article. Review.
-
Anticancer Effects of the Marine Sponge Lipastrotethya sp. Extract on Wild-Type and p53 Knockout HCT116 Cells.Evid Based Complement Alternat Med. 2017;2017:7174858. doi: 10.1155/2017/7174858. Epub 2017 Jan 3. Evid Based Complement Alternat Med. 2017. PMID: 28127380 Free PMC article.
-
Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition.Mar Drugs. 2024 Mar 23;22(4):143. doi: 10.3390/md22040143. Mar Drugs. 2024. PMID: 38667760 Free PMC article. Review.
References
-
- Wach J.-Y., Gademann K. Reduce to the maximum: Truncated natural products as powerful modulators of biological processes. Synlett. 2012;23:163–170.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

