Cyclic AMP-elevating Agents Promote Cumulus Cell Survival and Hyaluronan Matrix Stability, Thereby Prolonging the Time of Mouse Oocyte Fertilizability
- PMID: 26694612
- PMCID: PMC4759163
- DOI: 10.1074/jbc.M115.680983
Cyclic AMP-elevating Agents Promote Cumulus Cell Survival and Hyaluronan Matrix Stability, Thereby Prolonging the Time of Mouse Oocyte Fertilizability
Abstract
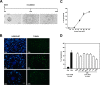
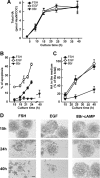
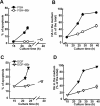
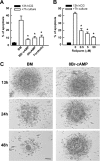
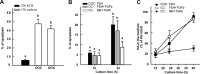
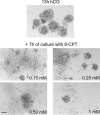
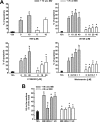
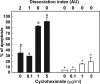
Cumulus cells sustain the development and fertilization of the mammalian oocyte. These cells are retained around the oocyte by a hyaluronan-rich extracellular matrix synthesized before ovulation, a process called cumulus cell-oocyte complex (COC) expansion. Hyaluronan release and dispersion of the cumulus cells progressively occur after ovulation, paralleling the decline of oocyte fertilization. We show here that, in mice, postovulatory changes of matrix are temporally correlated to cumulus cell death. Cumulus cell apoptosis and matrix disassembly also occurred in ovulated COCs cultured in vitro. COCs expanded in vitro with FSH or EGF underwent the same changes, whereas those expanded with 8-bromo-adenosine-3',5'-cyclic monophosphate (8-Br-cAMP) maintained integrity for a longer time. It is noteworthy that 8-Br-cAMP treatment was also effective on ovulated COCs cultured in vitro, prolonging the vitality of the cumulus cells and the stability of the matrix from a few hours to >2 days. Stimulation of endogenous adenylate cyclase with forskolin or inhibition of phosphodiesterase with rolipram produced similar effects. The treatment with selective cAMP analogues suggests that the effects of cAMP elevation are exerted through an EPAC-independent, PKA type II-dependent signaling pathway, probably acting at the post-transcriptional level. Finally, overnight culture of ovulated COCs with 8-Br-cAMP significantly counteracted the decrease of fertilization rate, doubling the number of fertilized oocytes compared with control conditions. In conclusion, these studies suggest that cAMP-elevating agents prevent cumulus cell senescence and allow them to continue to exert beneficial effects on oocyte and sperm, thereby extending in vitro the time frame of oocyte fertilizability.
Keywords: apoptosis; cumulus cells; cyclic AMP (cAMP); extracellular matrix; fertilization; hyaluronan.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization.Development. 2008 Jun;135(11):2001-11. doi: 10.1242/dev.020461. Epub 2008 Apr 23. Development. 2008. PMID: 18434414
-
FSH modulates PKAI and GPR3 activities in mouse oocyte of COC in a gap junctional communication (GJC)-dependent manner to initiate meiotic resumption.PLoS One. 2012;7(9):e37835. doi: 10.1371/journal.pone.0037835. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028418 Free PMC article.
-
FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte.Dev Biol. 1990 Mar;138(1):16-25. doi: 10.1016/0012-1606(90)90172-f. Dev Biol. 1990. PMID: 2155145
-
Molecular organization and mechanical properties of the hyaluronan matrix surrounding the mammalian oocyte.Matrix Biol. 2019 May;78-79:11-23. doi: 10.1016/j.matbio.2018.02.002. Epub 2018 Feb 9. Matrix Biol. 2019. PMID: 29408277 Review.
-
The Biological Role of Hyaluronan-Rich Oocyte-Cumulus Extracellular Matrix in Female Reproduction.Int J Mol Sci. 2018 Jan 18;19(1):283. doi: 10.3390/ijms19010283. Int J Mol Sci. 2018. PMID: 29346283 Free PMC article. Review.
Cited by
-
Peroxiredoxin 1 Controls Ovulation and Ovulated Cumulus-Oocyte Complex Activity through TLR4-Derived ERK1/2 Signaling in Mice.Int J Mol Sci. 2021 Aug 30;22(17):9437. doi: 10.3390/ijms22179437. Int J Mol Sci. 2021. PMID: 34502346 Free PMC article.
-
Expression pattern of olfactory receptor genes in human cumulus cells as an indicator for competent oocyte selection.Turk J Biol. 2020 Dec 14;44(6):371-380. doi: 10.3906/biy-2003-79. eCollection 2020. Turk J Biol. 2020. PMID: 33402864 Free PMC article.
-
Molecular Mechanisms of Action of FSH.Front Endocrinol (Lausanne). 2019 May 14;10:305. doi: 10.3389/fendo.2019.00305. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31139153 Free PMC article. Review.
-
Protein kinase A-mediated phosphorylation of the Broad-Complex transcription factor in silkworm suppresses its transcriptional activity.J Biol Chem. 2017 Jul 28;292(30):12460-12470. doi: 10.1074/jbc.M117.775130. Epub 2017 Jun 5. J Biol Chem. 2017. PMID: 28584058 Free PMC article.
-
The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility.Front Immunol. 2018 Nov 29;9:2808. doi: 10.3389/fimmu.2018.02808. eCollection 2018. Front Immunol. 2018. PMID: 30555480 Free PMC article. Review.
References
-
- Chian R. C., Lim J. H., and Tan S. L. (2004) State of the art in in-vitro oocyte maturation. Curr. Opin. Obstet. Gynecol. 16, 211–219 - PubMed
-
- Chang H. C., Liu H., Zhang J., Grifo J., and Krey L. C. (2005) Developmental incompetency of denuded mouse oocytes undergoing maturation in vitro is ooplasmic in nature and is associated with aberrant Oct-4 expression. Hum. Reprod. 20, 1958–1968 - PubMed
-
- Luciano A. M., Lodde V., Beretta M. S., Colleoni S., Lauria A., and Modina S. (2005) Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione. Mol. Reprod. Dev. 71, 389–397 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

