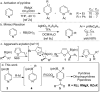
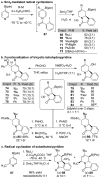
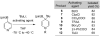Synthesis and Utility of Dihydropyridine Boronic Esters
- PMID: 26694785
- PMCID: PMC4769604
- DOI: 10.1002/anie.201510027
Synthesis and Utility of Dihydropyridine Boronic Esters
Abstract
When activated by an acylating agent, pyridine boronic esters react with organometallic reagents to form a dihydropyridine boronic ester. This intermediate allows access to a number of valuable substituted pyridine, dihydropyridine, and piperidine products.
Keywords: allylic compounds; boron; heterocycles; samarium iodide; synthetic methods.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Stereocontrolled synthesis of 1,5-stereogenic centers through three-carbon homologation of boronic esters.Angew Chem Int Ed Engl. 2014 Sep 8;53(37):9846-50. doi: 10.1002/anie.201405700. Epub 2014 Jul 15. Angew Chem Int Ed Engl. 2014. PMID: 25044518
-
Palladium-catalyzed, direct boronic acid synthesis from aryl chlorides: a simplified route to diverse boronate ester derivatives.J Am Chem Soc. 2010 Dec 22;132(50):17701-3. doi: 10.1021/ja1089759. Epub 2010 Nov 24. J Am Chem Soc. 2010. PMID: 21105666 Free PMC article.
-
Synthesis of Chiral Tertiary Boronic Esters: Phosphonate-Directed Catalytic Asymmetric Hydroboration of Trisubstituted Alkenes.J Am Chem Soc. 2017 May 3;139(17):6066-6069. doi: 10.1021/jacs.7b02324. Epub 2017 Apr 20. J Am Chem Soc. 2017. PMID: 28414243 Free PMC article.
-
Aminoboronic acids and esters: from synthetic challenges to the discovery of unique classes of enzyme inhibitors.Chem Soc Rev. 2011 Jul;40(7):3895-914. doi: 10.1039/c0cs00154f. Epub 2011 Mar 22. Chem Soc Rev. 2011. PMID: 21431144 Review.
-
Design and discovery of boronic acid drugs.Eur J Med Chem. 2020 Jun 1;195:112270. doi: 10.1016/j.ejmech.2020.112270. Epub 2020 Mar 30. Eur J Med Chem. 2020. PMID: 32302879 Review.
Cited by
-
Stereospecific 1,2-Migrations of Boronate Complexes Induced by Electrophiles.Angew Chem Int Ed Engl. 2020 Sep 21;59(39):16859-16872. doi: 10.1002/anie.202008096. Epub 2020 Jul 22. Angew Chem Int Ed Engl. 2020. PMID: 32592274 Free PMC article. Review.
-
Palladium Catalyzed Asymmetric Three-Component Coupling of Boronic Esters, Indoles, and Allylic Acetates.J Am Chem Soc. 2017 May 3;139(17):6038-6041. doi: 10.1021/jacs.7b01410. Epub 2017 Apr 21. J Am Chem Soc. 2017. PMID: 28414430 Free PMC article.
-
Cobalt-catalyzed double hydroboration of pyridines.Chem Sci. 2024 Mar 8;15(14):5201-5210. doi: 10.1039/d3sc05418g. eCollection 2024 Apr 3. Chem Sci. 2024. PMID: 38577376 Free PMC article.
-
Diastereoselective Alkylation of Activated Nitrogen Heterocycles with Alkenyl Boronate Complexes.Angew Chem Int Ed Engl. 2023 Apr 11;62(16):e202216961. doi: 10.1002/anie.202216961. Epub 2023 Mar 6. Angew Chem Int Ed Engl. 2023. PMID: 36780188 Free PMC article.
-
Chiral Aniline Synthesis via Stereospecific C(sp3)-C(sp2) Coupling of Boronic Esters with Aryl Hydrazines.Org Lett. 2018 Oct 5;20(19):6144-6147. doi: 10.1021/acs.orglett.8b02615. Epub 2018 Sep 17. Org Lett. 2018. PMID: 30221524 Free PMC article.
References
-
-
Reviews on addition to activated pyridines: Bull JA, Mousseau JJ, Pelletier G, Charette AB. Chem. Rev. 2012;112:2642–2713. Andersson H, Olsson R, Almqvist F. Org. Biomol. Chem. 2011;9:337–346. Comins DL, O'Connor S. In: Advances in Heterocyclic Chemistry. Alan RK, editor. Volume 44. Academic Press; 1988. pp. 199–267. Stout DM, Meyers AI. Chem. Rev. 1982;82:223–243.
-
-
-
For examples of Lewis acid-mediated addition to pyridines see Chen Q, León T, Knochel P. Angew. Chem. 2014;126:8891–8895. Angew. Chem. Int. Ed. 2014;53:8746–8750. Jeffrey JL, Sarpong R. Org. Lett. 2012;14:5400–5403.
-
-
-
For current examples of addition to activated pyridines see: Zhang P, Cook AM, Liu Y, Wolf CJ. Org. Chem. 2014;79:4167–4173. Tsukanov SV, Comins DL. Angew. Chem. 2011;123:8785–8787. Angew. Chem. Int. Ed. 2011;50:8626–8628.
-
-
- Chen Q, du Jourdin XM, Knochel P. J. Am. Chem. Soc. 2013;135:4958–4961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources