Small heat shock proteins are induced during multiple sclerosis lesion development in white but not grey matter
- PMID: 26694816
- PMCID: PMC4688967
- DOI: 10.1186/s40478-015-0267-2
Small heat shock proteins are induced during multiple sclerosis lesion development in white but not grey matter
Abstract
Introduction: The important protective role of small heat-shock proteins (HSPs) in regulating cellular survival and migration, counteracting protein aggregation, preventing apoptosis, and regulating inflammation in the central nervous system is now well-recognized. Yet, their role in the neuroinflammatory disorder multiple sclerosis (MS) is largely undocumented. With the exception of alpha B-crystallin (HSPB5), little is known about the roles of small HSPs in disease.
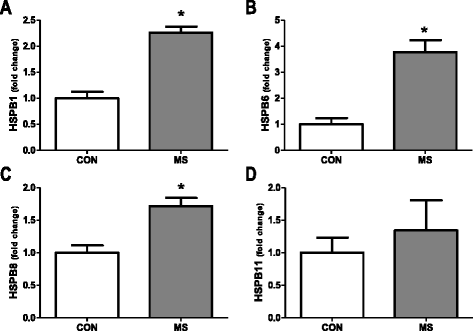
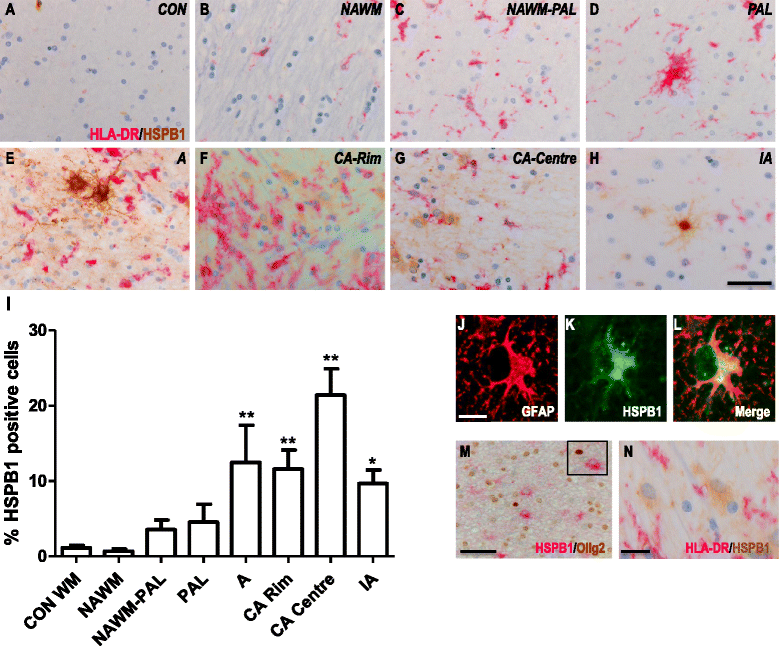
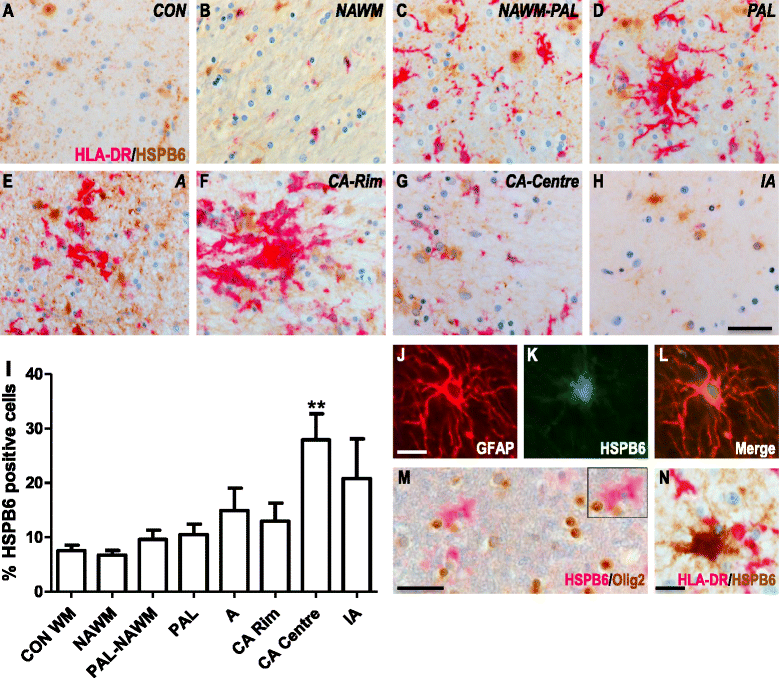
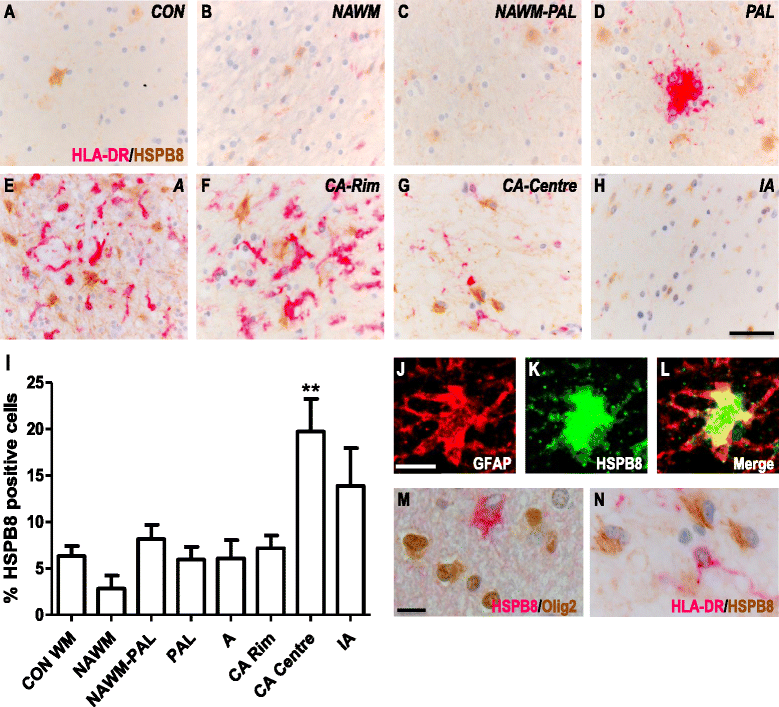
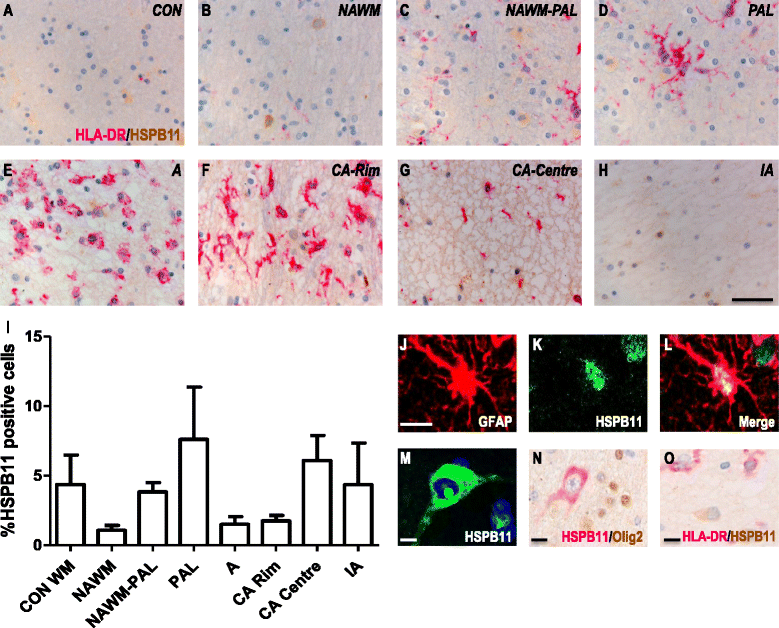
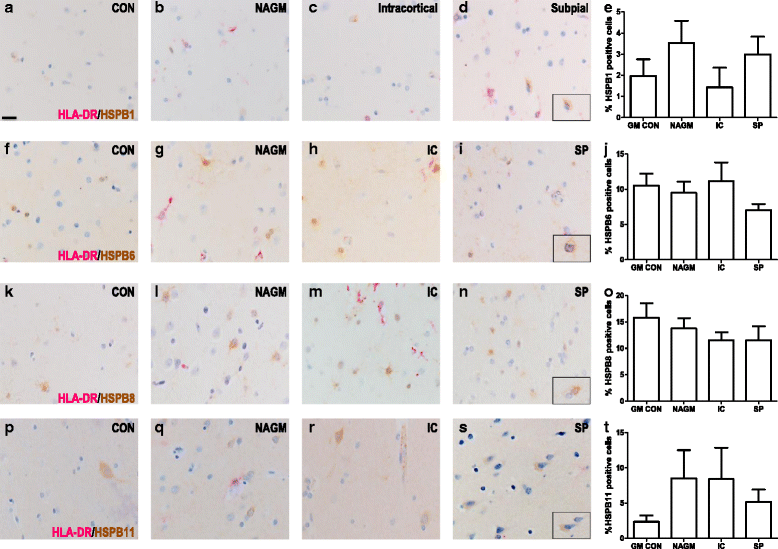
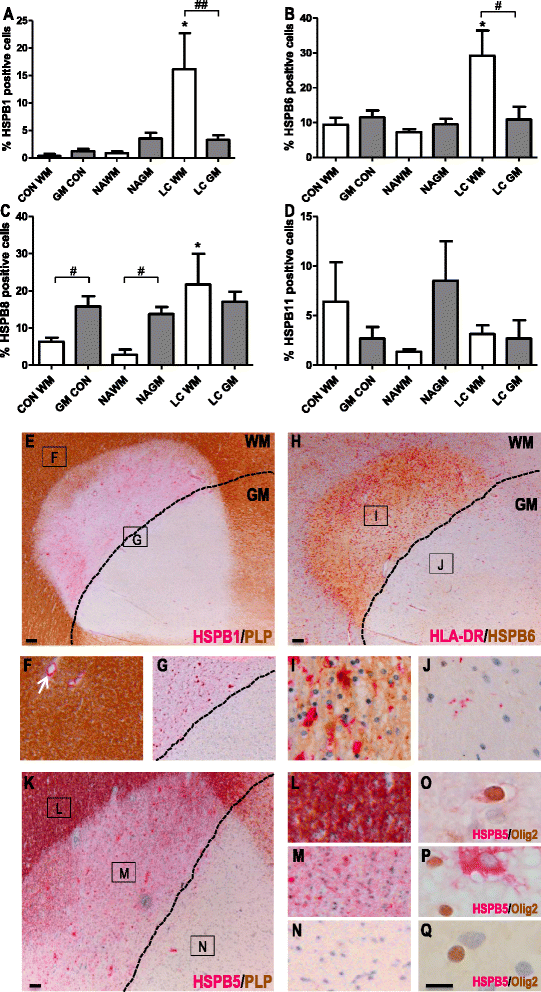
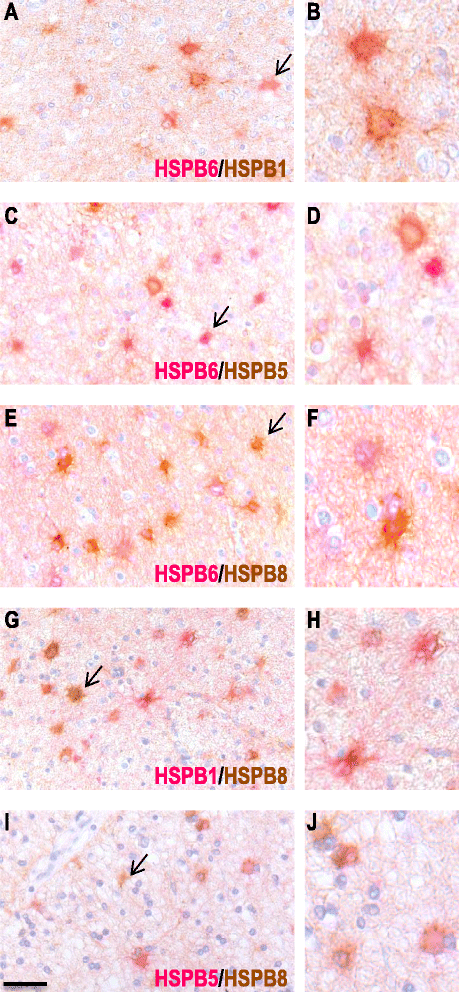
Results: Here, we examined the expression of four small HSPs during lesion development in MS, focussing on their cellular distribution, and regional differences between white matter (WM) and grey matter (GM). It is well known that MS lesions in these areas differ markedly in their pathology, with substantially more intense blood-brain barrier damage, leukocyte infiltration and microglial activation typifying WM but not GM lesions. We analysed transcript levels and protein distribution profiles for HSPB1, HSPB6, HSPB8 and HSPB11 in MS lesions at different stages, comparing them with normal-appearing brain tissue from MS patients and non-neurological controls. During active stages of demyelination in WM, and especially the centre of chronic active MS lesions, we found significantly increased expression of HSPB1, HSPB6 and HSPB8, but not HSPB11. When induced, small HSPs were exclusively found in astrocytes but not in oligodendrocytes, microglia or neurons. Surprisingly, while the numbers of astrocytes displaying high expression of small HSPs were markedly increased in actively demyelinating lesions in WM, no such induction was observed in GM lesions. This difference was particularly obvious in leukocortical lesions covering both WM and GM areas.
Conclusions: Since induction of small HSPs in astrocytes is apparently a secondary response to damage, their differential expression between WM and GM likely reflects differences in mediators that accompany demyelination in either WM or GM during MS. Our findings also suggest that during MS, cortical structures fail to benefit from the protective actions of small HSPs.
Figures
Similar articles
-
Heat shock proteins are differentially expressed in brain and spinal cord: implications for multiple sclerosis.Clin Exp Immunol. 2018 Nov;194(2):137-152. doi: 10.1111/cei.13186. Epub 2018 Sep 19. Clin Exp Immunol. 2018. PMID: 30014472 Free PMC article.
-
Heat shock protein expression in cerebral X-linked adrenoleukodystrophy reveals astrocyte stress prior to myelin loss.Neuropathol Appl Neurobiol. 2018 Jun;44(4):363-376. doi: 10.1111/nan.12399. Epub 2017 Apr 26. Neuropathol Appl Neurobiol. 2018. PMID: 28319253
-
Neuroinflammatory component of gray matter pathology in multiple sclerosis.Ann Neurol. 2016 Nov;80(5):776-790. doi: 10.1002/ana.24791. Epub 2016 Oct 25. Ann Neurol. 2016. PMID: 27686563 Free PMC article.
-
Pathological differences between white and grey matter multiple sclerosis lesions.Ann N Y Acad Sci. 2015 Sep;1351:99-113. doi: 10.1111/nyas.12841. Epub 2015 Jul 22. Ann N Y Acad Sci. 2015. PMID: 26200258 Review.
-
The histopathology of grey matter demyelination in multiple sclerosis.Acta Neurol Scand Suppl. 2009;(189):51-7. doi: 10.1111/j.1600-0404.2009.01216.x. Acta Neurol Scand Suppl. 2009. PMID: 19566500 Review.
Cited by
-
Macrophage-specific autophagy-related gene HSPB8 is involved in the macrophage polarization in atherosclerosis.BMC Cardiovasc Disord. 2023 Mar 18;23(1):141. doi: 10.1186/s12872-023-03158-2. BMC Cardiovasc Disord. 2023. PMID: 36934244 Free PMC article.
-
The Role of Small Heat Shock Proteins in Protein Misfolding Associated Motoneuron Diseases.Int J Mol Sci. 2022 Oct 4;23(19):11759. doi: 10.3390/ijms231911759. Int J Mol Sci. 2022. PMID: 36233058 Free PMC article. Review.
-
TLR3 agonists induce fibronectin aggregation by activated astrocytes: a role of pro-inflammatory cytokines and fibronectin splice variants.Sci Rep. 2020 Jan 17;10(1):532. doi: 10.1038/s41598-019-57069-4. Sci Rep. 2020. PMID: 31953424 Free PMC article.
-
Integrating single-nucleus sequence profiling to reveal the transcriptional dynamics of Alzheimer's disease, Parkinson's disease, and multiple sclerosis.J Transl Med. 2023 Sep 21;21(1):649. doi: 10.1186/s12967-023-04516-6. J Transl Med. 2023. PMID: 37735671 Free PMC article.
-
MAPK pathway and B cells overactivation in multiple sclerosis revealed by phosphoproteomics and genomic analysis.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9671-9676. doi: 10.1073/pnas.1818347116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004050 Free PMC article.
References
-
- Richter-Landsberg C. Heat shock proteins in neural cells. New York: Springer; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

