Physical and functional interaction between the α- and γ-secretases: A new model of regulated intramembrane proteolysis
- PMID: 26694839
- PMCID: PMC4687875
- DOI: 10.1083/jcb.201502001
Physical and functional interaction between the α- and γ-secretases: A new model of regulated intramembrane proteolysis
Abstract
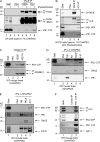
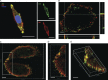
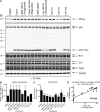
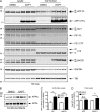
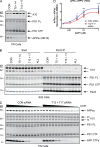
Many single-transmembrane proteins are sequentially cleaved by ectodomain-shedding α-secretases and the γ-secretase complex, a process called regulated intramembrane proteolysis (RIP). These cleavages are thought to be spatially and temporally separate. In contrast, we provide evidence for a hitherto unrecognized multiprotease complex containing both α- and γ-secretase. ADAM10 (A10), the principal neuronal α-secretase, interacted and cofractionated with γ-secretase endogenously in cells and mouse brain. A10 immunoprecipitation yielded γ-secretase proteolytic activity and vice versa. In agreement, superresolution microscopy showed that portions of A10 and γ-secretase colocalize. Moreover, multiple γ-secretase inhibitors significantly increased α-secretase processing (r = -0.86) and decreased β-secretase processing of β-amyloid precursor protein. Select members of the tetraspanin web were important both in the association between A10 and γ-secretase and the γ → α feedback mechanism. Portions of endogenous BACE1 coimmunoprecipitated with γ-secretase but not A10, suggesting that β- and α-secretases can form distinct complexes with γ-secretase. Thus, cells possess large multiprotease complexes capable of sequentially and efficiently processing transmembrane substrates through a spatially coordinated RIP mechanism.
© 2015 Chen et al.
Figures




References
-
- Buxbaum J.D., Liu K.N., Luo Y., Slack J.L., Stocking K.L., Peschon J.J., Johnson R.S., Castner B.J., Cerretti D.P., and Black R.A.. 1998. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 273:27765–27767. 10.1074/jbc.273.43.27765 - DOI - PubMed
-
- Cacquevel M., Aeschbach L., Osenkowski P., Li D., Ye W., Wolfe M.S., Li H., Selkoe D.J., and Fraering P.C.. 2008. Rapid purification of active γ-secretase, an intramembrane protease implicated in Alzheimer’s disease. J. Neurochem. 104:210–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

