XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses
- PMID: 26694969
- PMCID: PMC4710197
- DOI: 10.1084/jem.20142350
XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses
Abstract
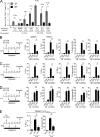
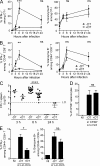
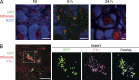
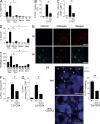
Naive CD8(+) T cell priming during tumor development or many primary infections requires cross-presentation by XCR1(+) dendritic cells (DCs). Memory CD8(+) T lymphocytes (mCTLs) harbor a lower activation threshold as compared with naive cells. However, whether their recall responses depend on XCR1(+) DCs is unknown. By using a new mouse model allowing fluorescent tracking and conditional depletion of XCR1(+) DCs, we demonstrate a differential requirement of these cells for mCTL recall during secondary infections by different pathogens. XCR1(+) DCs were instrumental to promote this function upon secondary challenges with Listeria monocytogenes, vesicular stomatitis virus, or Vaccinia virus, but dispensable in the case of mouse cytomegalovirus. We deciphered how XCR1(+) DCs promote mCTL recall upon secondary infections with Listeria. By visualizing for the first time the in vivo choreography of XCR1(+) DCs, NK cells and mCTLs during secondary immune responses, and by neutralizing in vivo candidate molecules, we demonstrate that, very early after infection, mCTLs are activated, and attracted in a CXCR3-dependent manner, by NK cell-boosted, IL-12-, and CXCL9-producing XCR1(+) DCs. Hence, depending on the infectious agent, strong recall of mCTLs during secondary challenges can require cytokine- and chemokine-dependent cross-talk with XCR1(+) DCs and NK cells.
© 2016 Alexandre et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

