Haptoglobin Preserves Vascular Nitric Oxide Signaling during Hemolysis
- PMID: 26694989
- PMCID: PMC4872667
- DOI: 10.1164/rccm.201510-2058OC
Haptoglobin Preserves Vascular Nitric Oxide Signaling during Hemolysis
Abstract
Rationale: Hemolysis occurs not only in conditions such as sickle cell disease and malaria but also during transfusion of stored blood, extracorporeal circulation, and sepsis. Cell-free Hb depletes nitric oxide (NO) in the vasculature, causing vasoconstriction and eventually cardiovascular complications. We hypothesize that Hb-binding proteins may preserve vascular NO signaling during hemolysis.
Objectives: Characterization of an archetypical function by which Hb scavenger proteins could preserve NO signaling during hemolysis.
Methods: We investigated NO reaction kinetics, effects on arterial NO signaling, and tissue distribution of cell-free Hb and its scavenger protein complexes.
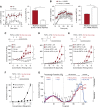
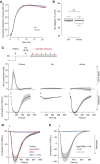
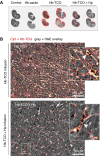
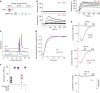
Measurements and main results: Extravascular translocation of cell-free Hb into interstitial spaces, including the vascular smooth muscle cell layer of rat and pig coronary arteries, promotes vascular NO resistance. This critical disease process is blocked by haptoglobin. Haptoglobin does not change NO dioxygenation rates of Hb; rather, the large size of the Hb:haptoglobin complex prevents Hb extravasation, which uncouples NO/Hb interaction and vasoconstriction. Size-selective compartmentalization of Hb functions as a substitute for red blood cells after hemolysis and preserves NO signaling in the vasculature. We found that evolutionarily and structurally unrelated Hb-binding proteins, such as PIT54 found in avian species, functionally converged with haptoglobin to protect NO signaling by sequestering cell-free Hb in large protein complexes.
Conclusions: Sequential compartmentalization of Hb by erythrocytes and scavenger protein complexes is an archetypical mechanism, which may have supported coevolution of hemolysis and normal vascular function. Therapeutic supplementation of Hb scavengers may restore vascular NO signaling and attenuate disease complications in patients with hemolysis.
Keywords: PIT54; extravasation; haptoglobin; hemoglobin; hemolysis.
Figures



Comment in
-
Compartmentalization Is Key in Limiting Nitric Oxide Scavenging by Cell-Free Hemoglobin.Am J Respir Crit Care Med. 2016 May 15;193(10):1072-4. doi: 10.1164/rccm.201512-2481ED. Am J Respir Crit Care Med. 2016. PMID: 27174473 Free PMC article. No abstract available.
Similar articles
-
Haptoglobin Therapeutics and Compartmentalization of Cell-Free Hemoglobin Toxicity.Trends Mol Med. 2020 Jul;26(7):683-697. doi: 10.1016/j.molmed.2020.02.004. Epub 2020 Mar 21. Trends Mol Med. 2020. PMID: 32589936 Review.
-
Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H743-54. doi: 10.1152/ajpheart.00151.2008. Epub 2008 Jun 13. Am J Physiol Heart Circ Physiol. 2008. PMID: 18552166 Free PMC article.
-
Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins.Blood. 2013 Feb 21;121(8):1276-84. doi: 10.1182/blood-2012-11-451229. Epub 2012 Dec 20. Blood. 2013. PMID: 23264591 Free PMC article. Review.
-
Endothelial dysfunction inhibits the ability of haptoglobin to prevent hemoglobin-induced hypertension.Am J Physiol Heart Circ Physiol. 2017 Jun 1;312(6):H1120-H1127. doi: 10.1152/ajpheart.00851.2016. Epub 2017 Mar 17. Am J Physiol Heart Circ Physiol. 2017. PMID: 28314763 Free PMC article.
-
Modular Platform for the Development of Recombinant Hemoglobin Scavenger Biotherapeutics.Mol Pharm. 2021 Aug 2;18(8):3158-3170. doi: 10.1021/acs.molpharmaceut.1c00433. Epub 2021 Jul 22. Mol Pharm. 2021. PMID: 34292741
Cited by
-
Hemolysis transforms liver macrophages into antiinflammatory erythrophagocytes.J Clin Invest. 2020 Oct 1;130(10):5576-5590. doi: 10.1172/JCI137282. J Clin Invest. 2020. PMID: 32663195 Free PMC article.
-
Blood-Related Toxicity after Traumatic Brain Injury: Potential Targets for Neuroprotection.Mol Neurobiol. 2020 Jan;57(1):159-178. doi: 10.1007/s12035-019-01766-8. Epub 2019 Oct 16. Mol Neurobiol. 2020. PMID: 31617072 Review.
-
Iron Regulation: Macrophages in Control.Pharmaceuticals (Basel). 2018 Dec 14;11(4):137. doi: 10.3390/ph11040137. Pharmaceuticals (Basel). 2018. PMID: 30558109 Free PMC article. Review.
-
Neurointerventional infusion of hemoglobin oxygen carrier prevents brain damage from acute cerebral ischemia in rats.Front Surg. 2023 Mar 3;10:1050935. doi: 10.3389/fsurg.2023.1050935. eCollection 2023. Front Surg. 2023. PMID: 36936654 Free PMC article.
-
Corticosterone, inflammation, immune status and telomere length in frigatebird nestlings facing a severe herpesvirus infection.Conserv Physiol. 2017 Jan 4;5(1):cow073. doi: 10.1093/conphys/cow073. eCollection 2017. Conserv Physiol. 2017. PMID: 28070333 Free PMC article.
References
-
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. - PubMed
-
- Vermeulen Windsant IC, de Wit NC, Sertorio JT, van Bijnen AA, Ganushchak YM, Heijmans JH, Tanus-Santos JE, Jacobs MJ, Maessen JG, Buurman WA. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

