Effects of a lifestyle program in subjects with Impaired Fasting Glucose, a pragmatic cluster-randomized controlled trial
- PMID: 26695176
- PMCID: PMC4688978
- DOI: 10.1186/s12875-015-0394-7
Effects of a lifestyle program in subjects with Impaired Fasting Glucose, a pragmatic cluster-randomized controlled trial
Abstract
Background: The worldwide epidemic of type 2 diabetes (T2DM) underlines the need for diabetes prevention strategies. In this study the feasibility and effectiveness of a nurse led lifestyle program for subjects with impaired fasting glucose (IFG) is assessed.
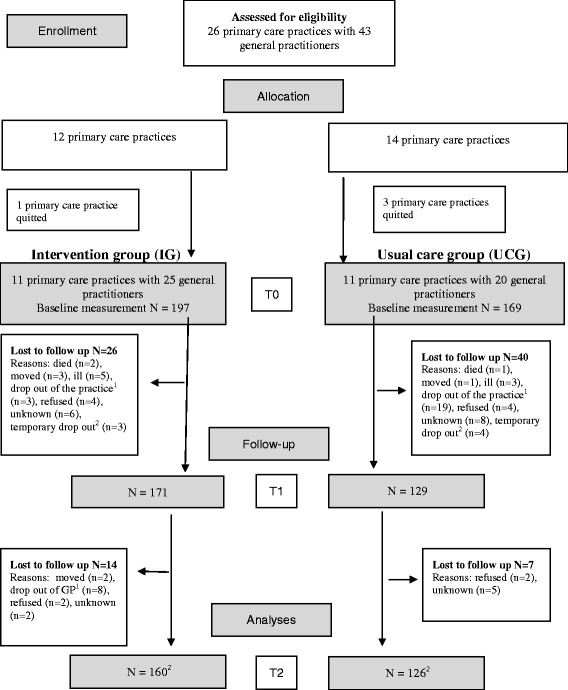
Methods: A cluster randomized clinical trial in 26 primary care practices in the Netherlands included 366 participants older than 45 years with newly diagnosed IFG and motivated to change their lifestyle (intervention group, n = 197; usual care group, n = 169). The one-year intervention, consisting of four to five individual nurse-led consultations, was directed at improving physical activity and dietary habits. The primary outcome measure was body mass index (BMI). Linear and logistic multilevel analyses and a process evaluation were performed.
Results: Both groups showed small reductions in BMI at 1 and 2 years, but differences between groups were not significant. At both 1 and 2-year follow-up the number of participants physically active for at least 30 minutes at least five days a week was significantly improved in the intervention group compared to the usual care group (intervention group vs. usual care group: OR1year = 3.53; 95 % CI = 1.69-7.37 and OR2years = 1.97; 95 % CI = 1.22-3.20, respectively). The total drop-out rate was 24 %. Process evaluation revealed that participants in the intervention group received fewer consultations than advised, while some practice nurses and participants considered the RM protocol too intensive.
Conclusions: This relatively simple lifestyle program in subjects with IFG resulted in a significant improvement in reported physical activity, but not in BMI. Despite its simplicity, some participants still considered the intervention too intensive. This viewpoint could be related to poor motivation and an absence of disease burden due to IFG, such that participants do not feel a need for behavioural change. Although the intervention provided some benefit, its wider use cannot be advised.
Trial registration: Current Controlled Trials ISRCTN41209683 , date of registration 16/10/2013h .
Figures
References
-
- Novoa FJ, Boronat M, Saavedra P, Díaz-Cremades JM, Varillas VF, Alberiche MP. Differences in cardiovascular risk factors, insulin resistance and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: the Telde Study. Diabetes Care. 2005;28:2388–2393. doi: 10.2337/diacare.28.10.2388. - DOI - PubMed
-
- Rutten G, de Grauw W, Nijpels G, Houweling B, van de Laar F, Bilo H, et al. NHG-Standaard ’Diabetes mellitus type 2’ (derde herziening). [Diabetes guideline of the Dutch College of General Practitioners. Third revision] Huisarts Wetenschap. 2013;56:512–525.
-
- Lindström J, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, Uusitupa M. Tuomilehto J; Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologica. 2013;56(2):284–293. doi: 10.1007/s00125-012-2752-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical