Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials
- PMID: 26695437
- PMCID: PMC5064722
- DOI: 10.1002/mds.26485
Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials
Abstract
Background: Continuous administration of levodopa-carbidopa intestinal gel (carbidopa-levodopa enteral suspension) through a percutaneous endoscopic gastrojejunostomy is a treatment option for advanced Parkinson disease (PD) patients with motor fluctuations resistant to standard oral medications. Safety data from 4 prospective studies were integrated to assess the safety of this therapy.
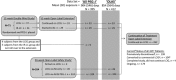
Methods: Safety data from 4 studies were summarized using 2 overlapping data sets, permitting the separation of procedure/device-associated (n = 395) from non-procedure/device adverse events (n = 412).
Results: At the data cutoff, median exposure to levodopa-carbidopa intestinal gel was 911 days (range, 1-1980 days) with 963 total patient-years of exposure. Procedure/device adverse events occurred in 300 patients (76%), and serious adverse events occurred in 68 (17%); most frequently reported procedure/device adverse events and serious adverse events were complications of device insertion (41% and 8%, respectively) and abdominal pain (36% and 4%, respectively). Non-procedure/device adverse events occurred in 92% (379), with most frequently reported being insomnia (23%) and falls (23%); 42% (171) had non-procedure/device serious adverse events, with most frequently reported being pneumonia (5%) and PD symptoms (2%). Adverse events led to discontinuation in 17% (72), most frequently because of complication of device insertion (2.4%). There were 34 treatment-emergent deaths (8.3%) in the overlapping data sets, 2 of which (0.5%) were considered "possibly related" to the treatment system.
Conclusion: In the largest collection of levodopa-carbidopa intestinal gel safety data from prospective clinical studies, procedure/device events were frequently reported and occasionally life threatening. Most non-procedure/device events were typical for levodopa treatment and an elderly population. These factors combined with high treatment efficacy led to a relatively low discontinuation rate in advanced PD patients.
Keywords: Levodopa-carbidopa intestinal gel; Parkinson's disease; infusion; percutaneous endoscopic gastrojejunostomy; safety.
© 2015 International Parkinson and Movement Disorder Society.
Figures

References
-
- Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16(3):448‐458. - PubMed
-
- Coelho M, Ferreira JJ. Late‐stage Parkinson disease. Nat Rev Neurol 2012;8(8):435‐442. - PubMed
-
- Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol 2010;257(Suppl 2):S253‐S261. - PubMed
-
- Nutt JG. Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 2008;23(Suppl 3):S580‐S584. - PubMed
-
- Quinn NP. Classification of fluctuations in patients with Parkinson's disease. Neurology 1998;51(2 Suppl 2):S25‐S29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

