Alcohol and Viral Hepatitis: Role of Lipid Rafts
- PMID: 26695752
- PMCID: PMC4590625
Alcohol and Viral Hepatitis: Role of Lipid Rafts
Abstract
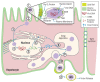
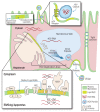
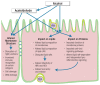
Both alcohol abuse and infection with hepatitis viruses can lead to liver disease, including chronic hepatitis. Alcohol and hepatitis viruses have synergistic effects in the development of liver disease. Some of these involve the cellular membranes and particularly their functionally active domains, termed lipid rafts, which contain many proteins with essential roles in signaling and other processes. These lipid rafts play a central role in the lifecycles of hepatitis viruses. Alcohol's actions at the lipid rafts may contribute to the synergistic harmful effects of alcohol and hepatitis viruses on the liver and the pathogenesis of liver disease.
Figures
Similar articles
-
Viruses and alcohol in the pathogenesis of primary hepatic carcinoma.Eur J Cancer Prev. 2001 Feb;10(1):107-10. doi: 10.1097/00008469-200102000-00016. Eur J Cancer Prev. 2001. PMID: 11263585 Review. No abstract available.
-
[Chronic active hepatitis].Rev Prat. 1995 Dec 15;45(20):2519-22. Rev Prat. 1995. PMID: 8578144 French. No abstract available.
-
Hepatitis C and alcohol: fundamental and translational research directions.Alcohol Clin Exp Res. 2003 Apr;27(4):726-31. doi: 10.1097/01.ALC.0000062741.58464.19. Alcohol Clin Exp Res. 2003. PMID: 12711937 Review.
-
[Immunohistologic findings in the liver, spleen, brain and skeletal muscles in fatal cholestatic viral hepatitis (following double infection with hepatitis B and non-A/non-B?)].Dtsch Z Verdau Stoffwechselkr. 1983;43(4):173-84. Dtsch Z Verdau Stoffwechselkr. 1983. PMID: 6414798 German.
-
Hepatitis virus and glomerulonephritis.Intern Med. 1998 Oct;37(10):800-1. doi: 10.2169/internalmedicine.37.800. Intern Med. 1998. PMID: 9840698 No abstract available.
Cited by
-
Effects of alcohol consumption on viral hepatitis B and C.World J Clin Cases. 2021 Nov 26;9(33):10052-10063. doi: 10.12998/wjcc.v9.i33.10052. World J Clin Cases. 2021. PMID: 34904075 Free PMC article. Review.
-
Effect of Low-Dose Alcohol Consumption on Chronic Liver Disease.Nutrients. 2024 Feb 23;16(5):613. doi: 10.3390/nu16050613. Nutrients. 2024. PMID: 38474740 Free PMC article. Review.
-
Anti-Fibrotic Effect of Oleamide Identified from the Moringa oleifera Lam. Leaves via Inhibition of TGF-β1-Induced SMAD2/3 Signaling Pathway.Int J Mol Sci. 2025 Apr 4;26(7):3388. doi: 10.3390/ijms26073388. Int J Mol Sci. 2025. PMID: 40244247 Free PMC article.
-
Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy.Toxicol Rep. 2021 Feb 19;8:376-385. doi: 10.1016/j.toxrep.2021.02.010. eCollection 2021. Toxicol Rep. 2021. PMID: 33680863 Free PMC article. Review.
-
SQSTM1/p62 and Hepatic Mallory-Denk Body Formation in Alcohol-Associated Liver Disease.Am J Pathol. 2023 Oct;193(10):1415-1426. doi: 10.1016/j.ajpath.2023.02.015. Epub 2023 Mar 9. Am J Pathol. 2023. PMID: 36906265 Free PMC article. Review.
References
-
- Adachi Y, Bradford BU, Gao W, et al. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–460. - PubMed
-
- Aliche-Djoudi F, Podechard N, Chevanne M, et al. Physical and chemical modulation of lipid rafts by a dietary n-3 polyunsaturated fatty acid increases ethanol-induced oxidative stress. Free Radical Biology & Medicine. 2011;51(11):2018–2030. - PubMed
-
- American Association for the Study of Liver Diseases. Recommendations for Testing, Managing, and Treating Hepatitis C. 2015. [Accessed May 5, 2015]. Available at: http://www.hcvguidelines.org/full-report-view. - PubMed
-
- Barboza L, Salmen S, Teran-Angel G, et al. A deficient translocation of CD3zeta, ZAP-70 and Grb2 to lipid raft, as a hallmark of defective adaptive immune response during chronic hepatitis B infection. Cellular Immunology. 2013;284(1–2):9–19. - PubMed
-
- Bartosch B, Vitelli A, Granier C, et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. Journal of Biological Chemistry. 2003;278(43):41624–41630. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

