Quinic acid derivatives inhibit dengue virus replication in vitro
- PMID: 26695767
- PMCID: PMC4688969
- DOI: 10.1186/s12985-015-0443-9
Quinic acid derivatives inhibit dengue virus replication in vitro
Abstract
Background: Dengue is the most prevalent arboviral disease in tropical and sub-tropical areas of the world. The incidence of infection is estimated to be 390 million cases and 25,000 deaths per year. Despite these numbers, neither a specific treatment nor a preventive vaccine is available to protect people living in areas of high risk.
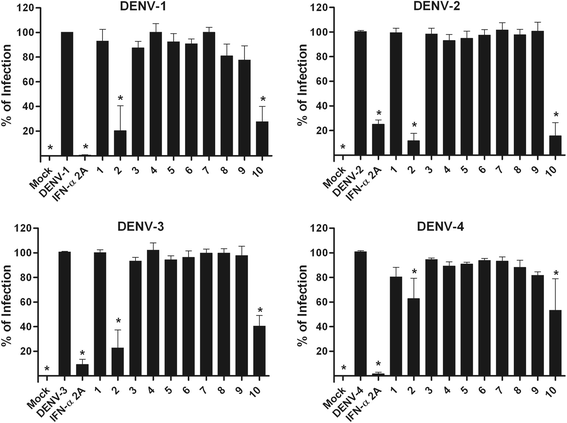
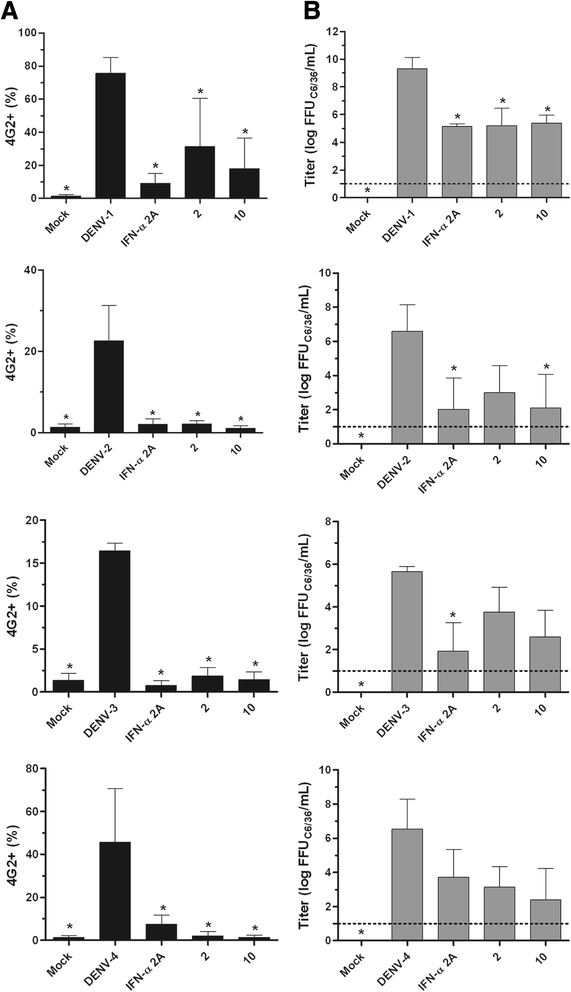
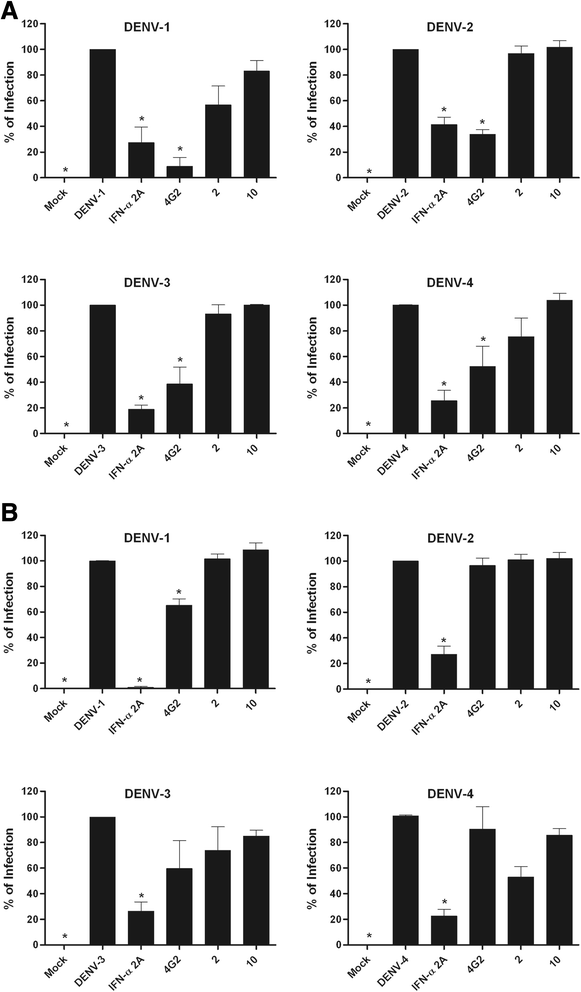
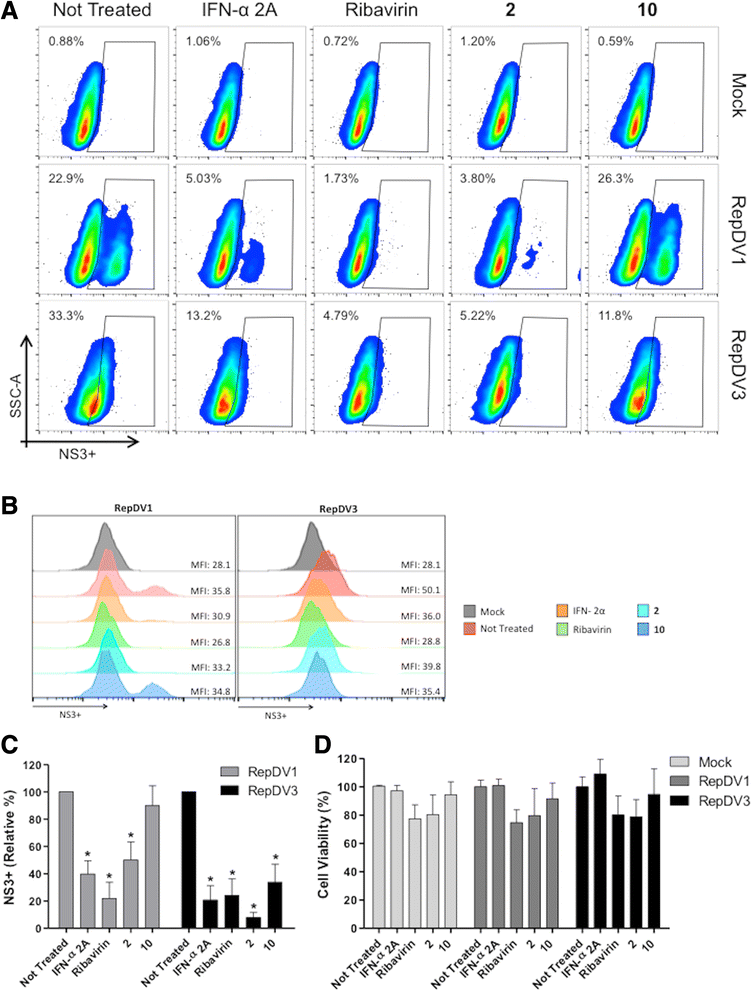
Results: With the aim of seeking a treatment that can mitigate dengue infection, we demonstrated that the quinic acid derivatives known as compound 2 and compound 10 were effective against all four dengue virus serotypes and safe for use in a human hepatoma cell line (Huh7.5). Both compounds were non-virucidal to dengue virus particles and did not interfere with early steps of the dengue virus life cycle, including binding and internalization. Experiments using a replicon system demonstrated that compounds 2 and 10 impaired dengue virus replication in Huh7.5 cells. Additionally, the anti-dengue virus effects of the quinic acid derivatives were preserved in human peripheral blood mononuclear cells.
Conclusions: Taken together, these data suggest that quinic acid derivatives represent a novel chemical class of active compounds that could be used to combat dengue virus infection.
Figures


Similar articles
-
Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity.Antiviral Res. 2014 Jun;106:125-34. doi: 10.1016/j.antiviral.2014.03.014. Epub 2014 Mar 27. Antiviral Res. 2014. PMID: 24680954 Free PMC article.
-
A small molecule inhibitor of dengue virus type 2 protease inhibits the replication of all four dengue virus serotypes in cell culture.Virol J. 2015 Feb 8;12:16. doi: 10.1186/s12985-015-0248-x. Virol J. 2015. PMID: 25886260 Free PMC article.
-
The citrus flavanone naringenin impairs dengue virus replication in human cells.Sci Rep. 2017 Feb 3;7:41864. doi: 10.1038/srep41864. Sci Rep. 2017. PMID: 28157234 Free PMC article.
-
Dengue Virus Reporter Replicon is a Valuable Tool for Antiviral Drug Discovery and Analysis of Virus Replication Mechanisms.Viruses. 2016 May 5;8(5):122. doi: 10.3390/v8050122. Viruses. 2016. PMID: 27164125 Free PMC article. Review.
-
The search for nucleoside/nucleotide analog inhibitors of dengue virus.Antiviral Res. 2015 Oct;122:12-9. doi: 10.1016/j.antiviral.2015.07.010. Epub 2015 Aug 1. Antiviral Res. 2015. PMID: 26241002 Review.
Cited by
-
In Vitro Digestion of Peanut Skin Releases Bioactive Compounds and Increases Cancer Cell Toxicity.Antioxidants (Basel). 2023 Jun 28;12(7):1356. doi: 10.3390/antiox12071356. Antioxidants (Basel). 2023. PMID: 37507896 Free PMC article.
-
Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections-An In Vitro and In Silico Perspective.Molecules. 2021 Sep 23;26(19):5768. doi: 10.3390/molecules26195768. Molecules. 2021. PMID: 34641314 Free PMC article.
-
Phytochemical Characterization, Anti-Oxidant, Anti-Enzymatic and Cytotoxic Effects of Artemisia verlotiorum Lamotte Extracts: A New Source of Bioactive Agents.Molecules. 2022 Sep 10;27(18):5886. doi: 10.3390/molecules27185886. Molecules. 2022. PMID: 36144622 Free PMC article.
-
Phytochemicals, antioxidant, and antimicrobial activities of Opuntia stricta fruits peel.Open Vet J. 2024 Oct;14(10):2642-2650. doi: 10.5455/OVJ.2024.v14.i10.14. Epub 2024 Oct 31. Open Vet J. 2024. PMID: 39545202 Free PMC article.
-
In silico studies evidenced the role of structurally diverse plant secondary metabolites in reducing SARS-CoV-2 pathogenesis.Sci Rep. 2020 Nov 25;10(1):20584. doi: 10.1038/s41598-020-77602-0. Sci Rep. 2020. PMID: 33239694 Free PMC article.
References
-
- OMS. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition. France; WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland. 2009. - PubMed
-
- PAHO. General Information: Dengue. 2014. http://www.paho.org/hq/index.php?option=com_content&view=article&id=4493.... Access date: 11/12/2015.
-
- Kalitzky M, Borowski P. Molecular Biology of the Flavivirus, 1st edition. Horizon Bioscience, Norfolk, United Kindom, 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

