Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice
- PMID: 26695882
- PMCID: PMC4717274
- DOI: 10.1111/acel.12425
Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice
Abstract
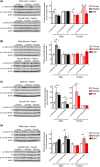
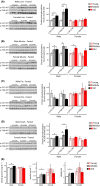
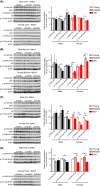
Inhibition of the mTOR (mechanistic Target Of Rapamycin) signaling pathway robustly extends the lifespan of model organisms including mice. The precise molecular mechanisms and physiological effects that underlie the beneficial effects of rapamycin are an exciting area of research. Surprisingly, while some data suggest that mTOR signaling normally increases with age in mice, the effect of age on mTOR signaling has never been comprehensively assessed. Here, we determine the age-associated changes in mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2) signaling in the liver, muscle, adipose, and heart of C57BL/6J.Nia mice, the lifespan of which can be extended by rapamycin treatment. We find that the effect of age on several different readouts of mTORC1 and mTORC2 activity varies by tissue and sex in C57BL/6J.Nia mice. Intriguingly, we observed increased mTORC1 activity in the liver and heart tissue of young female mice compared to male mice of the same age. Tissue and substrate-specific results were observed in the livers of HET3 and DBA/2 mouse strains, and in liver, muscle and adipose tissue of F344 rats. Our results demonstrate that aging does not result in increased mTOR signaling in most tissues and suggest that rapamycin does not promote lifespan by reversing or blunting such an effect.
Keywords: aging; mTOR; mTORC1; mTORC2; mice; rapamycin; sexual dimorphism.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
-
- Blagosklonny MV (2009) TOR‐driven aging: speeding car without brakes. Cell Cycle 8, 4055–4059. - PubMed
-
- Calhoun C, Shivshankar P, Saker M, Sloane LB, Livi CB, Sharp ZD, Orihuela CJ, Adnot S, White ES, Richardson A, Jourdan Le Saux C (2015) Senescent Cells Contribute to the Physiological Remodeling of Aged Lungs. J. Gerontol. A Biol. Sci. Med. Sci. Jan 7. pii: glu241. [Epub ahead of print] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

