Analysis of the interaction between host factor Sam68 and viral elements during foot-and-mouth disease virus infections
- PMID: 26695943
- PMCID: PMC4689063
- DOI: 10.1186/s12985-015-0452-8
Analysis of the interaction between host factor Sam68 and viral elements during foot-and-mouth disease virus infections
Abstract
Background: The nuclear protein Src-associated protein of 68 kDa in mitosis (Sam68) is known to bind RNA and be involved in cellular processes triggered in response to environmental stresses, including virus infection. Interestingly, Sam68 is a multi-functional protein implicated in the life cycle of retroviruses and picornaviruses and is also considered a marker of virus-induced stress granules (SGs). Recently, we demonstrated the partial redistribution of Sam68 to the cytoplasm in FMDV infected cells, its interaction with viral protease 3C(pro), and found a significant reduction in viral titers as consequence of Sam68-specific siRNA knockdowns. Despite of that, details of how it benefits FMDV remains to be elucidated.
Methods: Sam68 cytoplasmic localization was examined by immunofluorescent microscopy, counterstaining with antibodies against Sam68, a viral capsid protein and markers of SGs. The relevance of RAAA motifs in the IRES was investigated using electromobility shift assays with Sam68 protein and parental and mutant FMDV RNAs. In addition, full genome WT and mutant or G-luc replicon RNAs were tested following transfection in mammalian cells. The impact of Sam68 depletion to virus protein and RNA synthesis was investigated in a cell-free system. Lastly, through co-immunoprecipitation, structural modeling, and subcellular fractionation, viral protein interactions with Sam68 were explored.
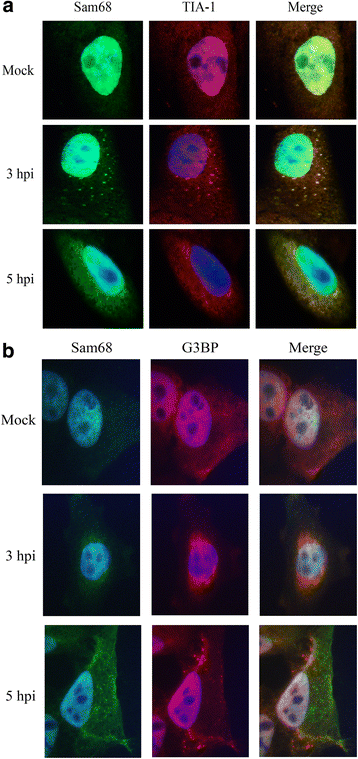
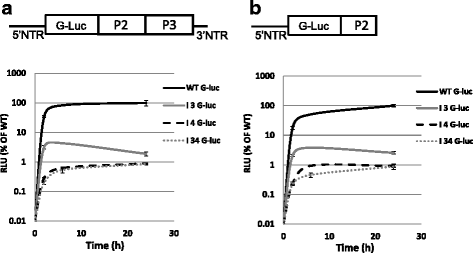
Results: FMDV-induced cytoplasmic redistribution of Sam68 resulted in it temporarily co-localizing with SG marker: TIA-1. Mutations that disrupted FMDV IRES RAAA motifs, with putative affinity to Sam68 in domain 3 and 4 cause a reduction on the formation of ribonucleoprotein complexes with this protein and resulted in non-viable progeny viruses and replication-impaired replicons. Furthermore, depletion of Sam68 in cell-free extracts greatly diminished FMDV RNA replication, which was restored by addition of recombinant Sam68. The results here demonstrated that Sam68 specifically co-precipitates with both FMDV 3D(pol) and 3C(pro) consistent with early observations of FMDV 3C(pro)-induced cleavage of Sam68.
Conclusion: We have found that Sam68 is a specific binding partner for FMDV non-structural proteins 3C(pro) and 3D(pol) and showed that mutations at RAAA motifs in IRES domains 3 and 4 cause a decrease in Sam68 affinity to these RNA elements and rendered the mutant RNA non-viable. Interestingly, in FMDV infected cells re-localized Sam68 was transiently detected along with SG markers in the cytoplasm. These results support the importance of Sam68 as a host factor co-opted by FMDV during infection and demonstrate that Sam68 interact with both, FMDV RNA motifs in the IRES and viral non-structural proteins 3C(pro) and 3D(pol).
Figures
Similar articles
-
The nuclear protein Sam68 is cleaved by the FMDV 3C protease redistributing Sam68 to the cytoplasm during FMDV infection of host cells.Virology. 2012 Mar 30;425(1):40-52. doi: 10.1016/j.virol.2011.12.019. Epub 2012 Jan 26. Virology. 2012. PMID: 22280896
-
Nuclear Protein Sam68 Interacts with the Enterovirus 71 Internal Ribosome Entry Site and Positively Regulates Viral Protein Translation.J Virol. 2015 Oct;89(19):10031-43. doi: 10.1128/JVI.01677-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202240 Free PMC article.
-
Heterogeneous Nuclear Ribonucleoprotein L Negatively Regulates Foot-and-Mouth Disease Virus Replication through Inhibition of Viral RNA Synthesis by Interacting with the Internal Ribosome Entry Site in the 5' Untranslated Region.J Virol. 2020 May 4;94(10):e00282-20. doi: 10.1128/JVI.00282-20. Print 2020 May 4. J Virol. 2020. PMID: 32161169 Free PMC article.
-
Biological function of Foot-and-mouth disease virus non-structural proteins and non-coding elements.Virol J. 2016 Jun 22;13:107. doi: 10.1186/s12985-016-0561-z. Virol J. 2016. PMID: 27334704 Free PMC article. Review.
-
Roles of the Picornaviral 3C Proteinase in the Viral Life Cycle and Host Cells.Viruses. 2016 Mar 17;8(3):82. doi: 10.3390/v8030082. Viruses. 2016. PMID: 26999188 Free PMC article. Review.
Cited by
-
The DDX23 Negatively Regulates Translation and Replication of Foot-and-Mouth Disease Virus and Is Degraded by 3C Proteinase.Viruses. 2020 Nov 25;12(12):1348. doi: 10.3390/v12121348. Viruses. 2020. PMID: 33255534 Free PMC article.
-
DDX21, a Host Restriction Factor of FMDV IRES-Dependent Translation and Replication.Viruses. 2021 Sep 3;13(9):1765. doi: 10.3390/v13091765. Viruses. 2021. PMID: 34578346 Free PMC article.
-
Host-Specific Interplay between Foot-and-Mouth Disease Virus 3D Polymerase and the Type-I Interferon Pathway.Viruses. 2023 Mar 1;15(3):666. doi: 10.3390/v15030666. Viruses. 2023. PMID: 36992375 Free PMC article.
-
Histone arginine demethylase JMJD6 is linked to stress granule assembly through demethylation of the stress granule-nucleating protein G3BP1.J Biol Chem. 2017 Nov 17;292(46):18886-18896. doi: 10.1074/jbc.M117.800706. Epub 2017 Sep 27. J Biol Chem. 2017. PMID: 28972166 Free PMC article.
-
Production of virus-like particles of FMDV by 3C protease cleaving precursor polyprotein P1 in vitro.Appl Microbiol Biotechnol. 2024 Dec 24;108(1):542. doi: 10.1007/s00253-024-13376-z. Appl Microbiol Biotechnol. 2024. PMID: 39718572 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

