Nascent osteoblast matrix inhibits osteogenesis of human mesenchymal stem cells in vitro
- PMID: 26696301
- PMCID: PMC4688995
- DOI: 10.1186/s13287-015-0223-x
Nascent osteoblast matrix inhibits osteogenesis of human mesenchymal stem cells in vitro
Abstract
Introduction: Adult mesenchymal stem cells (MSCs) are considered promising candidates for cell-based therapies. Their potential utility derives primarily from their immunomodulatory activity, multi-lineage differentiation potential, and likely progenitor cell function in wound healing and repair of connective tissues. However, in vitro, MSCs often senesce and spontaneously differentiate into osteoblasts after prolonged expansion, likely because of lack of regulatory microenvironmental signals. In vivo, osteoblasts that line the endosteal bone marrow surface are in close proximity to MSCs in the marrow stroma and thus may help to regulate MSC fate.
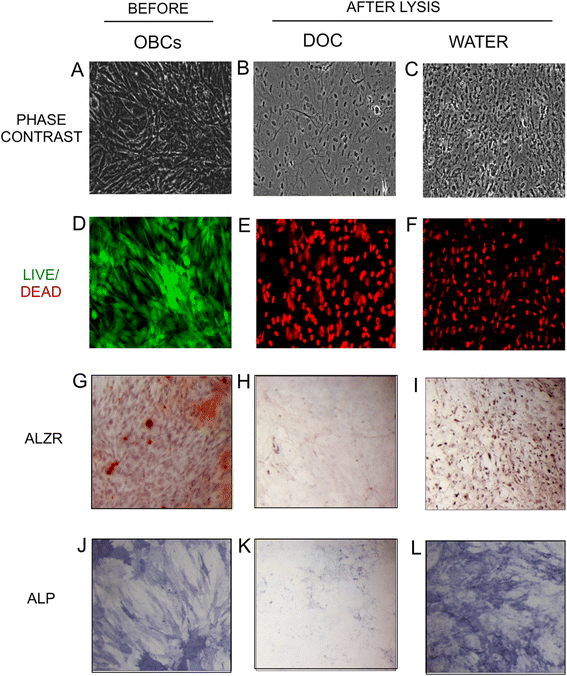
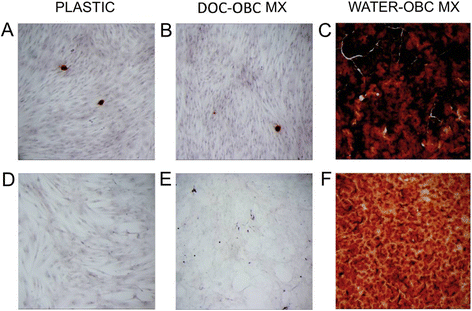
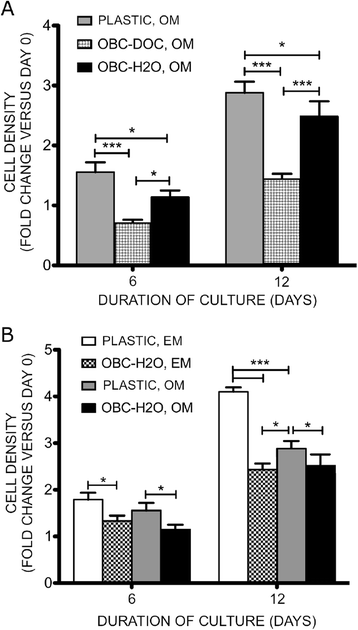
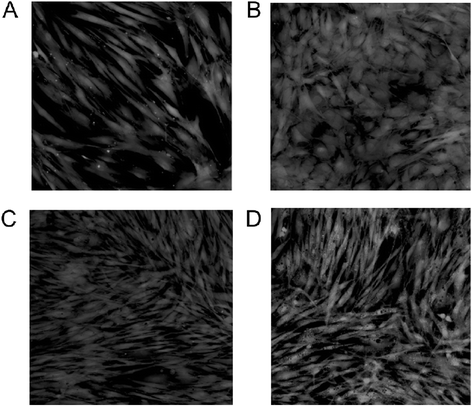
Methods: We examined here how osteogenic differentiation of MSCs in vitro is affected by exposure to osteoblastic cells (OBCs). Human bone marrow MSCs were exposed to OBCs, derived by induced osteogenic differentiation of MSCs, either directly in contact co-cultures, or indirectly to OBC-conditioned medium or decellularized OBC extracellular matrix (ECM).
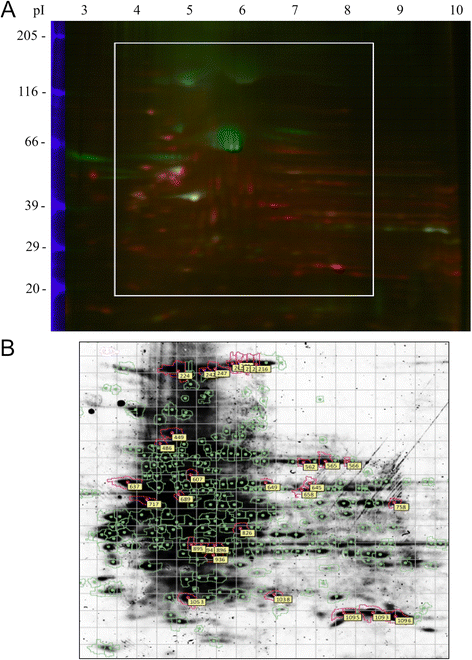
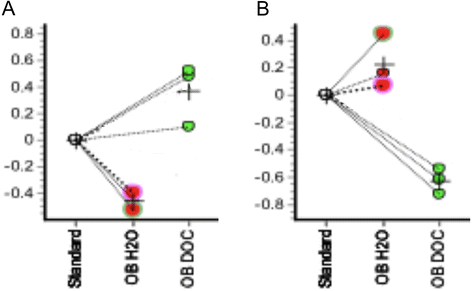
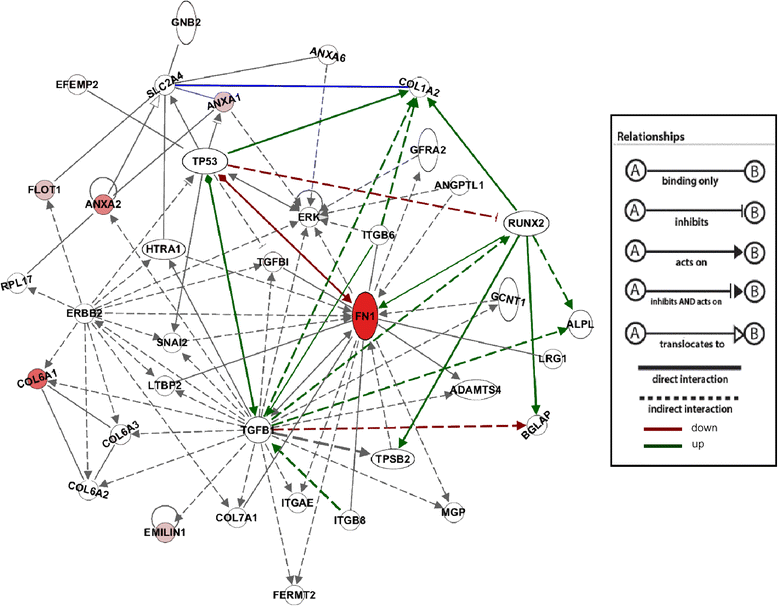
Results: Our results showed that OBCs can act as negative regulators of MSC osteogenesis. mRNA expression profiling revealed that OBCs did not affect MSC osteogenesis in direct contact cultures or via secreted factors. However, seeding MSCs on decellularized OBC ECM significantly decreased expression of several osteogenic genes and maintained their fibroblastic morphologies. Proteomic analysis identified some of the candidate protein regulators of MSC osteogenesis.
Conclusions: These findings provide the basis for future studies to elucidate the signaling mechanisms responsible for osteoblast matrix-mediated regulation of MSC osteogenesis and to better manipulate MSC fate in vitro to minimize their spontaneous differentiation.
Figures

Similar articles
-
Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche.Eur Cell Mater. 2012 Jan 12;23:13-27. doi: 10.22203/ecm.v023a02. Eur Cell Mater. 2012. PMID: 22241610
-
Osteogenic differentiation is synergistically influenced by osteoinductive treatment and direct cell-cell contact between murine osteoblasts and mesenchymal stem cells.Int Orthop. 2012 Jan;36(1):199-205. doi: 10.1007/s00264-011-1259-x. Epub 2011 May 13. Int Orthop. 2012. PMID: 21567150 Free PMC article.
-
Mesenchymal Stem Cells Repress Osteoblast Differentiation Under Osteogenic-Inducing Conditions.J Cell Biochem. 2015 Dec;116(12):2896-902. doi: 10.1002/jcb.25237. J Cell Biochem. 2015. PMID: 26013001
-
Adult mesenchymal stem cells: is there a role for purine receptors in their osteogenic differentiation?Purinergic Signal. 2020 Sep;16(3):263-287. doi: 10.1007/s11302-020-09703-4. Epub 2020 Jun 4. Purinergic Signal. 2020. PMID: 32500422 Free PMC article. Review.
-
Mechanism of Interleukin-17A Regulation of Mesenchymal Stroma/Stem Cell Osteogenic Differentiation.Discov Med. 2024 Dec;36(191):2343-2355. doi: 10.24976/Discov.Med.202436191.216. Discov Med. 2024. PMID: 39726309 Review.
Cited by
-
A New Chapter for Mesenchymal Stem Cells: Decellularized Extracellular Matrices.Stem Cell Rev Rep. 2017 Oct;13(5):587-597. doi: 10.1007/s12015-017-9757-x. Stem Cell Rev Rep. 2017. PMID: 28766102 Review.
-
SIRT1 was involved in TNF-α-promoted osteogenic differentiation of human DPSCs through Wnt/β-catenin signal.In Vitro Cell Dev Biol Anim. 2016 Dec;52(10):1001-1011. doi: 10.1007/s11626-016-0070-9. Epub 2016 Aug 16. In Vitro Cell Dev Biol Anim. 2016. PMID: 27530621
-
[Domestic artificial cervical disc interface pressure distribution and effect of bone-implant interface pressure on osseointegration].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017 Apr 15;31(4):443-450. doi: 10.7507/1002-1892.201610121. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017. PMID: 29798610 Free PMC article. Chinese.
-
Behavior and biocompatibility of rabbit bone marrow mesenchymal stem cells with bacterial cellulose membrane.PeerJ. 2018 Apr 30;6:e4656. doi: 10.7717/peerj.4656. eCollection 2018. PeerJ. 2018. PMID: 29736332 Free PMC article.
-
Use of allograft bone matrix in clinical orthopedics.Regen Med. 2024 May 3;19(5):247-256. doi: 10.1080/17460751.2024.2353473. Epub 2024 Jul 19. Regen Med. 2024. PMID: 39028538 Free PMC article. Review.
References
-
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

