New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia
- PMID: 26696796
- PMCID: PMC4678320
- DOI: 10.1159/000439002
New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia
Abstract
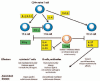
Autoimmune hemolytic anemia (AIHA) is caused by the increased destruction of red blood cells (RBCs) by anti-RBC autoantibodies with or without complement activation. RBC destruction may occur both by a direct lysis through the sequential activation of the final components of the complement cascade (membrane attack complex), or by antibody-dependent cell-mediated cytotoxicity (ADCC). The pathogenic role of autoantibodies depends on their class (the most frequent are IgG and IgM), subclass, thermal amplitude (warm and cold forms),as well as affinity and efficiency in activating complement. Several cytokines and cytotoxic mechanisms (CD8+ T and natural killer cells) are further involved in RBC destruction. Moreover, activated macrophages carrying Fc receptors may recognize and phagocyte erythrocytes opsonized by autoantibodies and complement. Direct complement-mediated lysis takes place mainly in the circulations and liver, whereas ADCC, cytotoxicity, and phagocytosis occur preferentially in the spleen and lymphoid organs. The degree of intravascular hemolysis is 10-fold greater than extravascular one. Finally, the efficacy of the erythroblastic compensatory response can greatly influence the clinical picture of AIHA. The interplay and relative burden of all these pathogenic mechanisms give reason for the great clinical heterogeneity of AIHAs, from fully compensated to rapidly evolving fatal cases.
Keywords: Autoantibodies; Autoimmune hemolytic anemia; Cytokines.
Figures
References
-
- Petz LD, Garratty G. Immune Hemolytic Anemias, 2nd ed. Philadelphia, Churchill Livingstone, 2004.
-
- Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309–317. - PubMed
-
- Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258–271. - PubMed
-
- Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology, Philadelphia, Saunders, 2012.
-
- McQueen F. A B cell explanation for autoimmune disease: the forbidden clone returns. Postgrad Med J. 2012;88:226–233. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

