Golgi Fragmentation in ALS Motor Neurons. New Mechanisms Targeting Microtubules, Tethers, and Transport Vesicles
- PMID: 26696811
- PMCID: PMC4672084
- DOI: 10.3389/fnins.2015.00448
Golgi Fragmentation in ALS Motor Neurons. New Mechanisms Targeting Microtubules, Tethers, and Transport Vesicles
Abstract
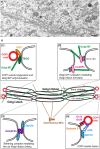
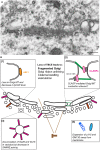
Pathological alterations of the Golgi apparatus, such as its fragmentation represent an early pre-clinical feature of many neurodegenerative diseases and have been widely studied in the motor neuron disease amyotrophic lateral sclerosis (ALS). Yet, the underlying molecular mechanisms have remained cryptic. In principle, Golgi fragmentation may result from defects in three major classes of proteins: structural Golgi proteins, cytoskeletal proteins and molecular motors, as well as proteins mediating transport to and through the Golgi. Here, we present the different mechanisms that may underlie Golgi fragmentation in animal and cellular models of ALS linked to mutations in SOD1, TARDBP (TDP-43), VAPB, and C9Orf72 and we propose a novel one based on findings in progressive motor neuronopathy (pmn) mice. These mice are mutated in the TBCE gene encoding the cis-Golgi localized tubulin-binding cofactor E, one of five chaperones that assist in tubulin folding and microtubule polymerization. Loss of TBCE leads to alterations in Golgi microtubules, which in turn impedes on the maintenance of the Golgi architecture. This is due to down-regulation of COPI coat components, dispersion of Golgi tethers and strong accumulation of ER-Golgi SNAREs. These effects are partially rescued by the GTPase ARF1 through recruitment of TBCE to the Golgi. We hypothesize that defects in COPI vesicles, microtubules and their interaction may also underlie Golgi fragmentation in human ALS linked to other mutations, spinal muscular atrophy (SMA), and related motor neuron diseases. We also discuss the functional relevance of pathological Golgi alterations, in particular their potential causative, contributory, or compensatory role in the degeneration of motor neuron cell bodies, axons and synapses.
Keywords: ALS; C9orf72; Golgi fragmentation; SOD1; TBCE; TDP-43; microtubules; neurodegeneration.
Figures
References
-
- Aguilera-Gomez A., Rabouille C. (2015). “Intra golgi transport,” Encyclopedia of Cell Biology, Vol. 2, eds Bradshaw R., Stahl P. (Amsterdam: Elsevier; ), 354–362. 10.1016/B978-0-12-394447-4.20034-5 - DOI
-
- Al-Sarraj S., King A., Troakes C., Smith B., Maekawa S., Bodi I., et al. . (2011). p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 122, 691–702. 10.1007/s00401-011-0911-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

