Paternal Retrieval Behavior Regulated by Brain Estrogen Synthetase (Aromatase) in Mouse Sires that Engage in Communicative Interactions with Pairmates
- PMID: 26696812
- PMCID: PMC4678232
- DOI: 10.3389/fnins.2015.00450
Paternal Retrieval Behavior Regulated by Brain Estrogen Synthetase (Aromatase) in Mouse Sires that Engage in Communicative Interactions with Pairmates
Abstract
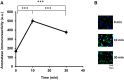
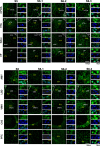
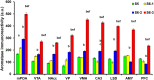
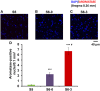
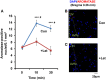
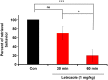
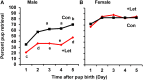
Parental behaviors involve complex social recognition and memory processes and interactive behavior with children that can greatly facilitate healthy human family life. Fathers play a substantial role in child care in a small but significant number of mammals, including humans. However, the brain mechanism that controls male parental behavior is much less understood than that controlling female parental behavior. Fathers of non-monogamous laboratory ICR mice are an interesting model for examining the factors that influence paternal responsiveness because sires can exhibit maternal-like parental care (retrieval of pups) when separated from their pups along with their pairmates because of olfactory and auditory signals from the dams. Here we tested whether paternal behavior is related to femininity by the aromatization of testosterone. For this purpose, we measured the immunoreactivity of aromatase [cytochrome P450 family 19 (CYP19)], which synthesizes estrogen from androgen, in nine brain regions of the sire. We observed higher levels of aromatase expression in these areas of the sire brain when they engaged in communicative interactions with dams in separate cages. Interestingly, the number of nuclei with aromatase immunoreactivity in sires left together with maternal mates in the home cage after pup-removing was significantly larger than that in sires housed with a whole family. The capacity of sires to retrieve pups was increased following a period of 5 days spent with the pups as a whole family after parturition, whereas the acquisition of this ability was suppressed in sires treated daily with an aromatase inhibitor. The results demonstrate that the dam significantly stimulates aromatase in the male brain and that the presence of the pups has an inhibitory effect on this increase. These results also suggest that brain aromatization regulates the initiation, development, and maintenance of paternal behavior in the ICR male mice.
Keywords: brain aromatase; communicative interaction; immunoreactivity; mouse; parental behavior; paternal care.
Figures
Similar articles
-
Pairmate-dependent pup retrieval as parental behavior in male mice.Front Neurosci. 2014 Jul 11;8:186. doi: 10.3389/fnins.2014.00186. eCollection 2014. Front Neurosci. 2014. PMID: 25071431 Free PMC article.
-
c-Fos expression in the paternal mouse brain induced by communicative interaction with maternal mates.Mol Brain. 2014 Sep 11;7:66. doi: 10.1186/s13041-014-0066-x. Mol Brain. 2014. PMID: 25208928 Free PMC article.
-
Displays of paternal mouse pup retrieval following communicative interaction with maternal mates.Nat Commun. 2013;4:1346. doi: 10.1038/ncomms2336. Nat Commun. 2013. PMID: 23299896 Free PMC article.
-
Hormones associated with non-maternal infant care: a review of mammalian and avian studies.Folia Primatol (Basel). 2000 Jan-Apr;71(1-2):6-21. doi: 10.1159/000021726. Folia Primatol (Basel). 2000. PMID: 10686483 Review.
-
Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain.J Steroid Biochem Mol Biol. 1997 Apr;61(3-6):365-74. J Steroid Biochem Mol Biol. 1997. PMID: 9365212 Review.
Cited by
-
Sex-Specific Differences in Oxytocin Receptor Expression and Function for Parental Behavior.Gend Genome. 2017 Dec 1;1(4):142-166. doi: 10.1089/gg.2017.0017. Gend Genome. 2017. PMID: 32959027 Free PMC article.
-
Sex and Pubertal Status Influence Dendritic Spine Density on Frontal Corticostriatal Projection Neurons in Mice.Cereb Cortex. 2020 May 18;30(6):3543-3557. doi: 10.1093/cercor/bhz325. Cereb Cortex. 2020. PMID: 32037445 Free PMC article.
-
Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex?Brain Res. 2017 Jan 1;1654(Pt B):123-144. doi: 10.1016/j.brainres.2016.08.042. Epub 2016 Aug 31. Brain Res. 2017. PMID: 27590721 Free PMC article. Review.
-
Molecular basis of autism spectrum disorders.Mol Biol Rep. 2025 May 28;52(1):508. doi: 10.1007/s11033-025-10604-1. Mol Biol Rep. 2025. PMID: 40434434 Free PMC article. Review.
-
Is paternal oxytocin an oxymoron? Oxytocin, vasopressin, testosterone, oestradiol and cortisol in emerging fatherhood.Philos Trans R Soc Lond B Biol Sci. 2022 Aug 29;377(1858):20210060. doi: 10.1098/rstb.2021.0060. Epub 2022 Jul 11. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 35858109 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

