Developmental Exposure to Ethinylestradiol Affects Reproductive Physiology, the GnRH Neuroendocrine Network and Behaviors in Female Mouse
- PMID: 26696819
- PMCID: PMC4673314
- DOI: 10.3389/fnins.2015.00463
Developmental Exposure to Ethinylestradiol Affects Reproductive Physiology, the GnRH Neuroendocrine Network and Behaviors in Female Mouse
Abstract
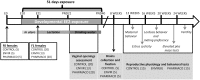
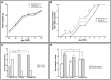
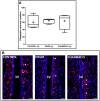
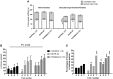
During development, environmental estrogens are able to induce an estrogen mimetic action that may interfere with endocrine and neuroendocrine systems. The present study investigated the effects on the reproductive function in female mice following developmental exposure to pharmaceutical ethinylestradiol (EE2), the most widespread and potent synthetic steroid present in aquatic environments. EE2 was administrated in drinking water at environmentally relevant (ENVIR) or pharmacological (PHARMACO) doses [0.1 and 1 μg/kg (body weight)/day respectively], from embryonic day 10 until postnatal day 40. Our results show that both groups of EE2-exposed females had advanced vaginal opening and shorter estrus cycles, but a normal fertility rate compared to CONTROL females. The hypothalamic population of GnRH neurons was affected by EE2 exposure with a significant increase in the number of perikarya in the preoptic area of the PHARMACO group and a modification in their distribution in the ENVIR group, both associated with a marked decrease in GnRH fibers immunoreactivity in the median eminence. In EE2-exposed females, behavioral tests highlighted a disturbed maternal behavior, a higher lordosis response, a lack of discrimination between gonad-intact and castrated males in sexually experienced females, and an increased anxiety-related behavior. Altogether, these results put emphasis on the high sensitivity of sexually dimorphic behaviors and neuroendocrine circuits to disruptive effects of EDCs.
Keywords: GnRH; endocrine disruption; ethinylestradiol; neuroendocrinology; reproduction; sexual behavior.
Figures

Similar articles
-
Developmental exposure to Ethinylestradiol affects transgenerationally sexual behavior and neuroendocrine networks in male mice.Sci Rep. 2015 Dec 7;5:17457. doi: 10.1038/srep17457. Sci Rep. 2015. PMID: 26640081 Free PMC article.
-
Developmental exposure of zebrafish (Danio rerio) to 17α-ethinylestradiol affects non-reproductive behavior and fertility as adults, and increases anxiety in unexposed progeny.Horm Behav. 2015 Jul;73:30-8. doi: 10.1016/j.yhbeh.2015.05.014. Epub 2015 Jun 10. Horm Behav. 2015. PMID: 26072466
-
Altered non-reproductive behavior and feminization caused by developmental exposure to 17α-ethinylestradiol persist to adulthood in three-spined stickleback (Gasterosteus aculeatus).Aquat Toxicol. 2019 Feb;207:142-152. doi: 10.1016/j.aquatox.2018.11.024. Epub 2018 Nov 28. Aquat Toxicol. 2019. PMID: 30572174
-
Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications.Brain Res Brain Res Rev. 2001 Nov;37(1-3):178-200. doi: 10.1016/s0165-0173(01)00118-7. Brain Res Brain Res Rev. 2001. PMID: 11744086 Review.
-
Neuroendocrine and behavioral implications of endocrine disrupting chemicals in quail.Horm Behav. 2001 Sep;40(2):234-47. doi: 10.1006/hbeh.2001.1695. Horm Behav. 2001. PMID: 11534988 Review.
Cited by
-
Effects of gestational low dose perfluorooctanoic acid on maternal and "anxiety-like" behavior in dams.Front Toxicol. 2022 Aug 29;4:971970. doi: 10.3389/ftox.2022.971970. eCollection 2022. Front Toxicol. 2022. PMID: 36105436 Free PMC article.
-
Cocktails of NSAIDs and 17α Ethinylestradiol at Environmentally Relevant Doses in Drinking Water Alter Puberty Onset in Mice Intergenerationally.Int J Mol Sci. 2023 Mar 20;24(6):5890. doi: 10.3390/ijms24065890. Int J Mol Sci. 2023. PMID: 36982971 Free PMC article.
-
Developmental exposure to low levels of ethinylestradiol affects social play in juvenile male rats.Toxicol Res. 2020 Feb 7;36(4):301-310. doi: 10.1007/s43188-019-00035-z. eCollection 2020 Oct. Toxicol Res. 2020. PMID: 33005589 Free PMC article.
-
Integrated Genomic and Bioinformatics Approaches to Identify Molecular Links between Endocrine Disruptors and Adverse Outcomes.Int J Environ Res Public Health. 2022 Jan 5;19(1):574. doi: 10.3390/ijerph19010574. Int J Environ Res Public Health. 2022. PMID: 35010832 Free PMC article. Review.
-
Developmental estrogen exposures and disruptions to maternal behavior and brain: Effects of ethinyl estradiol, a common positive control.Horm Behav. 2018 May;101:113-124. doi: 10.1016/j.yhbeh.2017.10.013. Epub 2017 Nov 8. Horm Behav. 2018. PMID: 29107581 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

