Generation of Functional Neuromuscular Junctions from Human Pluripotent Stem Cell Lines
- PMID: 26696831
- PMCID: PMC4672046
- DOI: 10.3389/fncel.2015.00473
Generation of Functional Neuromuscular Junctions from Human Pluripotent Stem Cell Lines
Abstract
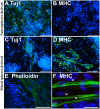
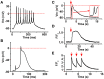
Several neuromuscular diseases involve dysfunction of neuromuscular junctions (NMJs), yet there are no patient-specific human models for electrophysiological characterization of NMJ. We seeded cells of neurally-induced embryoid body-like spheres derived from induced pluripotent stem cell (iPSC) or embryonic stem cell (ESC) lines as monolayers without basic fibroblast factor (bFGF) and observed differentiation of neuronal as well as spontaneously contracting, multinucleated skeletal myotubes. The myotubes showed striation, immunoreactivity for myosin heavy chain, actin bundles typical for myo-oriented cells, and generated spontaneous and evoked action potentials (APs). The myogenic differentiation was associated with expression of MyoD1, myogenin and type I ryanodine receptor. Neurons formed end plate like structures with strong binding of α-bungarotoxin, a marker of nicotinic acetylcholine receptors highly expressed in the postsynaptic membrane of NMJs, and expressed SMI-32, a motoneuron marker, as well as SV2, a marker for synapses. Pharmacological stimulation of cholinergic receptors resulted in strong depolarization of myotube membrane and raised Ca(2+) concentration in sarcoplasm, while electrical stimulation evoked Ca(2+) transients in myotubes. Stimulation of motoneurons with N-Methyl-D-aspartate resulted in reproducible APs in myotubes and end plates displayed typical mEPPs and tonic activity depolarizing myotubes of about 10 mV. We conclude that simultaneous differentiation of neurons and myotubes from patient-specific iPSCs or ESCs results also in the development of functional NMJs. Our human model of NMJ may serve as an important tool to investigate normal development, mechanisms of diseases and novel drug targets involving NMJ dysfunction and degeneration.
Keywords: cell culture; embryonic; human; induced pluripotent stem cell; nerve cell engineering; skeletal muscle.
Figures




Similar articles
-
Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes.Stem Cell Res. 2015 Sep;15(2):328-36. doi: 10.1016/j.scr.2015.07.005. Epub 2015 Jul 26. Stem Cell Res. 2015. PMID: 26255853
-
Simplified in vitro engineering of neuromuscular junctions between rat embryonic motoneurons and immortalized human skeletal muscle cells.Stem Cells Cloning. 2019 Feb 20;12:1-9. doi: 10.2147/SCCAA.S187655. eCollection 2019. Stem Cells Cloning. 2019. PMID: 30863121 Free PMC article.
-
Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived-From the Same Induced Pluripotent Stem Cell Source.Bioengineering (Basel). 2020 Oct 22;7(4):133. doi: 10.3390/bioengineering7040133. Bioengineering (Basel). 2020. PMID: 33105732 Free PMC article.
-
Neuromuscular synapse electrophysiology in myasthenia gravis animal models.Ann N Y Acad Sci. 2018 Jan;1412(1):146-153. doi: 10.1111/nyas.13507. Epub 2017 Oct 25. Ann N Y Acad Sci. 2018. PMID: 29068559 Review.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
Cited by
-
Tissue-Engineered Skeletal Muscle Models to Study Muscle Function, Plasticity, and Disease.Front Physiol. 2021 Feb 26;12:619710. doi: 10.3389/fphys.2021.619710. eCollection 2021. Front Physiol. 2021. PMID: 33716768 Free PMC article. Review.
-
A Human-Based Functional NMJ System for Personalized ALS Modeling and Drug Testing.Adv Ther (Weinh). 2020 Nov;3(11):2000133. doi: 10.1002/adtp.202000133. Epub 2020 Aug 11. Adv Ther (Weinh). 2020. PMID: 33709015 Free PMC article.
-
On-chip, multisite extracellular and intracellular recordings from primary cultured skeletal myotubes.Sci Rep. 2016 Nov 4;6:36498. doi: 10.1038/srep36498. Sci Rep. 2016. PMID: 27812002 Free PMC article.
-
Human mesenchymal amniotic fluid stem cells reveal an unexpected neuronal potential differentiating into functional spinal motor neurons.Front Cell Dev Biol. 2022 Jul 22;10:936990. doi: 10.3389/fcell.2022.936990. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938174 Free PMC article.
-
A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits.Biomaterials. 2019 Dec;225:119537. doi: 10.1016/j.biomaterials.2019.119537. Epub 2019 Oct 8. Biomaterials. 2019. PMID: 31614290 Free PMC article.
References
-
- Boltze J., Kleinschnitz C., Reymann K. G., Reiser G., Wagner D. C., Kranz A., et al. . (2012). Neurovascular pathophysiology in cerebral ischemia, dementia and the ageing brain-current trends in basic, translational and clinical research. Exp. Transl. Stroke Med. 4:14. 10.1186/2040-7378-4-14 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

