Endothelial Barrier and Its Abnormalities in Cardiovascular Disease
- PMID: 26696899
- PMCID: PMC4673665
- DOI: 10.3389/fphys.2015.00365
Endothelial Barrier and Its Abnormalities in Cardiovascular Disease
Abstract
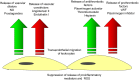
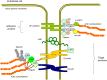
Endothelial cells (ECs) form a unique barrier between the vascular lumen and the vascular wall. In addition, the endothelium is highly metabolically active. In cardiovascular disease such as atherosclerosis and hypertension, normal endothelial function could be severely disturbed leading to endothelial dysfunction that then could progress to complete and irreversible loss of EC functionality and contribute to entire vascular dysfunction. Proatherogenic stimuli such as diabetes, dyslipidemia, and oxidative stress could initiate endothelial dysfunction and in turn vascular dysfunction and lead to the development of atherosclerotic arterial disease, a background for multiple cardiovascular disorders including coronary artery disease, acute coronary syndrome, stroke, and thrombosis. Intercellular junctions between ECs mediate the barrier function. Proinflammatory stimuli destabilize the junctions causing the disruption of the endothelial barrier and increased junctional permeability. This facilitates transendothelial migration of immune cells to the arterial intima and induction of vascular inflammation. Proatherogenic stimuli attack endothelial microtubule function that is regulated by acetylation of tubulin, an essential microtubular constituent. Chemical modification of tubulin caused by cardiometabolic risk factors and oxidative stress leads to reorganization of endothelial microtubules. These changes destabilize vascular integrity and increase permeability, which finally results in increasing cardiovascular risk.
Keywords: cardiovascular disease; cell-to-cell junctions; endothelial barrier; endothelial intercellular junctions; endothelium.
Figures


Similar articles
-
Endothelial dysfunction as a cellular mechanism for vascular failure.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H499-505. doi: 10.1152/ajpheart.00325.2011. Epub 2011 Nov 11. Am J Physiol Heart Circ Physiol. 2012. PMID: 22081698 Review.
-
Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review.Cardiovasc Res. 2018 Jan 1;114(1):35-52. doi: 10.1093/cvr/cvx226. Cardiovasc Res. 2018. PMID: 29228169 Free PMC article. Review.
-
Crossing the wall: the opening of endothelial cell junctions during infectious diseases.Int J Biochem Cell Biol. 2013 Jul;45(7):1165-73. doi: 10.1016/j.biocel.2013.03.010. Epub 2013 Mar 26. Int J Biochem Cell Biol. 2013. PMID: 23538297 Review.
-
The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability.Am J Respir Cell Mol Biol. 2003 May;28(5):574-81. doi: 10.1165/rcmb.2002-0075OC. Am J Respir Cell Mol Biol. 2003. PMID: 12707013
-
Vascular endothelial growth factor-D is overexpressed in human cardiac allograft vasculopathy and diabetic atherosclerosis and induces endothelial permeability to low-density lipoproteins in vitro.J Heart Lung Transplant. 2011 Aug;30(8):955-62. doi: 10.1016/j.healun.2011.04.007. Epub 2011 May 28. J Heart Lung Transplant. 2011. PMID: 21620738
Cited by
-
The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study.Int J Mol Sci. 2023 Feb 2;24(3):2930. doi: 10.3390/ijms24032930. Int J Mol Sci. 2023. PMID: 36769252 Free PMC article.
-
Systematic Bioinformatic Analyses of Nutrigenomic Modifications by Polyphenols Associated with Cardiometabolic Health in Humans-Evidence from Targeted Nutrigenomic Studies.Nutrients. 2021 Jul 6;13(7):2326. doi: 10.3390/nu13072326. Nutrients. 2021. PMID: 34371836 Free PMC article.
-
The Superantigen Toxic Shock Syndrome Toxin 1 Alters Human Aortic Endothelial Cell Function.Infect Immun. 2018 Feb 20;86(3):e00848-17. doi: 10.1128/IAI.00848-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29229737 Free PMC article.
-
Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation.Oxid Med Cell Longev. 2021 Feb 11;2021:6651367. doi: 10.1155/2021/6651367. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628373 Free PMC article. Review.
-
Extracellular Vesicles in Periodontitis: Pathogenic Mechanisms and Therapeutic Potential.J Inflamm Res. 2025 Jan 28;18:1317-1331. doi: 10.2147/JIR.S504612. eCollection 2025. J Inflamm Res. 2025. PMID: 39897520 Free PMC article. Review.
References
-
- Babinska A., Azari B. M., Salifu M. O., Liu R., Jiang X. C., Sobocka M. B., et al. . (2007). The F11 receptor (F11R/JAM-A) in atherothrombosis: overexpression of F11R in atherosclerotic plaques. Thromb. Haemost. 97, 272–281. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

