Elastic and Muscular Arteries Differ in Structure, Basal NO Production and Voltage-Gated Ca(2+)-Channels
- PMID: 26696904
- PMCID: PMC4678217
- DOI: 10.3389/fphys.2015.00375
Elastic and Muscular Arteries Differ in Structure, Basal NO Production and Voltage-Gated Ca(2+)-Channels
Abstract
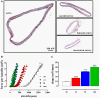
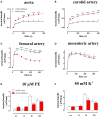
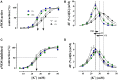
In the last decades, the search for mechanisms underlying progressive arterial stiffening and for interventions to avoid or reverse this process has gained much attention. In general, arterial stiffening displays regional variation and is, for example, during aging more prominent in elastic than in muscular arteries. We hypothesize that besides passive also active regulators of arterial compliance [i.e., endothelial and vascular smooth muscle cell (VSMC) function] differ between these arteries. Hence, it is conceivable that these vessel types will display different time frames of stiffening. To investigate this hypothesis segments of muscular arteries such as femoral and mesenteric arteries and elastic arteries such as the aorta and carotid artery were isolated from female C57Bl6 mice (5-6 months of age, n = 8). Both microscopy and passive stretching of the segments in a myograph confirmed that passive mechanical properties (elastin, collagen) of elastic and muscular arteries were significantly different. Endothelial function, more specifically basal nitric oxide (NO) efficacy, and VSMC function, more specifically L-type voltage-gated Ca(2+) channel (VGCC)-mediated contractions, were determined by α1-adrenoceptor stimulation with phenylephrine (PE) and by gradual depolarization with elevated extracellular K(+) in the absence and presence of eNOS inhibition with L-NAME. PE-mediated isometric contractions significantly increased after inhibition of NO release with L-NAME in elastic, but not in muscular vessel segments. This high basal eNOS activity in elastic vessels was also responsible for shifts of K(+) concentration-contraction curves to higher external K(+). VGCC-mediated contractions were similarly affected by depolarization with elevated K(+) in muscular artery segments or in elastic artery segments in the absence of basal NO. However, K(+)-induced contractions were inhibited by the VGCC blocker diltiazem with significantly higher sensitivity in the muscular arteries, suggestive of different populations of VGCC isoforms in both vessel types. The results from the present study demonstrate that, besides passive arterial wall components, also active functional components contribute to the heterogeneity of arterial compliance along the vascular tree. This crucially facilitates the search for (patho) physiological mechanisms and potential therapeutic targets to treat or reverse large artery stiffening as occurring in aging-induced arterial stiffening.
Keywords: arterial compliance; arterial stiffness; basal nitric oxide; diltiazem; elastic arteries; muscular arteries; voltage-gated calcium channels.
Figures

References
-
- Borlotti A., Khir A. W., Rietzschel E. R., De Buyzere M. L., Vermeersch S., Segers P. (2012). Noninvasive determination of local pulse wave velocity and wave intensity: changes with age and gender in the carotid and femoral arteries of healthy human. J. Appl. Physiol. (1985) 113, 727–735. 10.1152/japplphysiol.00164.2012 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

