Voltage-Sensitive K(+) Channels Inhibit Parasympathetic Ganglion Transmission and Vagal Control of Heart Rate in Hypertensive Rats
- PMID: 26696959
- PMCID: PMC4672051
- DOI: 10.3389/fneur.2015.00260
Voltage-Sensitive K(+) Channels Inhibit Parasympathetic Ganglion Transmission and Vagal Control of Heart Rate in Hypertensive Rats
Abstract
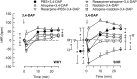
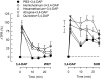
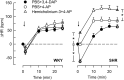
Parasympathetic withdrawal plays an important role in the autonomic dysfunctions in hypertension. Since hyperpolarizing, voltage-sensitive K(+) channels (K V) hamper transmitter release, elevated K V-activity may explain the disturbed vagal control of heart rate (HR) in hypertension. Here, the K V inhibitor 3,4-diaminopyridine was used to demonstrate the impact of K V on autonomic HR control. Cardiac output and HR were recorded by a flow probe on the ascending aorta in anesthetized, normotensive (WKY), and spontaneously hypertensive rats (SHR), and blood pressure by a femoral artery catheter. 3,4-diaminopyridine induced an initial bradycardia, which was greater in SHR than in WKY, followed by sustained tachycardia in both strains. The initial bradycardia was eliminated by acetylcholine synthesis inhibitor (hemicholinium-3) and nicotinic receptor antagonist/ganglion blocker (hexamethonium), and reversed to tachycardia by muscarinic receptor (mAchR) antagonist (atropine). The latter was abolished by sympatho-inhibition (reserpine). Reserpine also eliminated the late, 3,4-diaminopyridine-induced tachycardia in WKY, but induced a sustained atropine-sensitive bradycardia in SHR. Inhibition of the parasympathetic component with hemicholinium-3, hexamethonium, or atropine enhanced the late tachycardia in SHR, whereas hexamethonium reduced the tachycardia in WKY. In conclusion, 3,4-diaminopyridine-induced acetylcholine release, and thus enhanced parasympathetic ganglion transmission, with subsequent mAchR activation and bradycardia. 3,4-diaminopyridine also activated tachycardia, initially by enhancing sympathetic ganglion transmission, subsequently by activation of norepinephrine release from sympathetic nerve terminals. The 3,4-diaminopyridine-induced parasympathetic activation was stronger and more sustained in SHR, demonstrating an enhanced inhibitory control of K V on parasympathetic ganglion transmission. This enhanced K V activity may explain the dysfunctional vagal HR control in SHR.
Keywords: 3,4-diaminopyridine; acetylcholine release; heart rate; hypertension; norepinephrine release; parasympathetic ganglia; sympathetic ganglia; voltage-sensitive K+-channels.
Figures


Similar articles
-
M-currents (Kv7.2-7.3/KCNQ2-KCNQ3) Are Responsible for Dysfunctional Autonomic Control in Hypertensive Rats.Front Physiol. 2016 Nov 29;7:584. doi: 10.3389/fphys.2016.00584. eCollection 2016. Front Physiol. 2016. PMID: 27965589 Free PMC article.
-
Simultaneous parasympathetic and sympathetic activation reveals altered autonomic control of heart rate, vascular tension, and epinephrine release in anesthetized hypertensive rats.Front Neurol. 2011 Nov 23;2:71. doi: 10.3389/fneur.2011.00071. eCollection 2011. Front Neurol. 2011. PMID: 22131984 Free PMC article.
-
Balance among autonomic controls of heart rate in neonatal spontaneously hypertensive and borderline hypertensive rats.J Auton Nerv Syst. 1988 Feb;22(1):11-21. doi: 10.1016/0165-1838(88)90149-x. J Auton Nerv Syst. 1988. PMID: 2895129
-
A unique central cholinergic deficit in the spontaneously hypertensive rat: physostigmine reveals a bradycardia associated with sensory stimulation.J Pharmacol Exp Ther. 1994 Mar;268(3):1081-90. J Pharmacol Exp Ther. 1994. PMID: 8138921
-
Central cardiovascular effects of dihydropyridines in spontaneously hypertensive rats.Fundam Clin Pharmacol. 1989;3 Suppl:47s-56s. doi: 10.1111/j.1472-8206.1989.tb00474.x. Fundam Clin Pharmacol. 1989. PMID: 2693290 Review.
Cited by
-
M-currents (Kv7.2-7.3/KCNQ2-KCNQ3) Are Responsible for Dysfunctional Autonomic Control in Hypertensive Rats.Front Physiol. 2016 Nov 29;7:584. doi: 10.3389/fphys.2016.00584. eCollection 2016. Front Physiol. 2016. PMID: 27965589 Free PMC article.
-
The Role of Sympathetic Nerves in Osteoporosis: A Narrative Review.Biomedicines. 2022 Dec 23;11(1):33. doi: 10.3390/biomedicines11010033. Biomedicines. 2022. PMID: 36672541 Free PMC article. Review.
-
The Kv7 Channel and Cardiovascular Risk Factors.Front Cardiovasc Med. 2017 Dec 5;4:75. doi: 10.3389/fcvm.2017.00075. eCollection 2017. Front Cardiovasc Med. 2017. PMID: 29259974 Free PMC article. Review.
-
Musings on the wanderer: What's new in our understanding of vago-vagal reflexes? VI. Central vagal circuits that control glucose metabolism.Am J Physiol Gastrointest Liver Physiol. 2021 Jan 1;320(2):G175-G182. doi: 10.1152/ajpgi.00368.2020. Epub 2020 Nov 18. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33205998 Free PMC article. Review.
References
-
- Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep (2009) 11:199–205. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

