Polyamine and Its Metabolite H2O2 Play a Key Role in the Conversion of Embryogenic Callus into Somatic Embryos in Upland Cotton (Gossypium hirsutum L.)
- PMID: 26697030
- PMCID: PMC4667013
- DOI: 10.3389/fpls.2015.01063
Polyamine and Its Metabolite H2O2 Play a Key Role in the Conversion of Embryogenic Callus into Somatic Embryos in Upland Cotton (Gossypium hirsutum L.)
Abstract
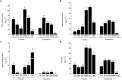
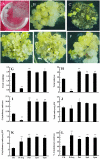
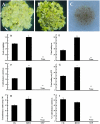
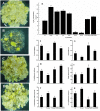
The objective of this study was to increase understanding about the mechanism by which polyamines (PAs) promote the conversion of embryogenic calli (EC) into somatic embryos in cotton (Gossypium hirsutum L.). We measured the levels of endogenous PAs and H2O2, quantified the expression levels of genes involved in the PAs pathway at various stages of cotton somatic embryogenesis (SE), and investigated the effects of exogenous PAs and H2O2 on differentiation and development of EC. Putrescine (Put), spermidine (Spd), and spermine (Spm) significantly increased from the EC stage to the early phase of embryo differentiation. The levels of Put then decreased until the somatic embryo stage whereas Spd and Spm remained nearly the same. The expression profiles of GhADC genes were consistent with changes in Put during cotton SE. The H2O2 concentrations began to increase significantly at the EC stage, during which time both GhPAO1 and GhPAO4 expressions were highest and PAO activity was significantly increased. Exogenous Put, Spd, Spm, and H2O2 not only enhanced embryogenic callus growth and embryo formation, but also alleviated the effects of D-arginine and 1, 8-diamino-octane, which are inhibitors of PA synthesis and PAO activity. Overall, the results suggest that both PAs and their metabolic product H2O2 are essential for the conversion of EC into somatic embryos in cotton.
Keywords: hydrogen peroxide (H2O2); nitric oxide (NO); polyamine oxidase (PAO); polyamines (PAs); somatic embryogenesis (SE); upland cotton.
Figures







References
-
- Alscher R. G., Donahue J. L., Cramer C. L. (1997). Reactive oxygen species and antioxidants: relationships in green cells. Physiol. Plant. 100 224–233. 10.1111/j.1399-3054.1997.tb04778.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

