Human Amnion-Derived Mesenchymal Stem Cells Promote Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells by Influencing the ERK1/2 Signaling Pathway
- PMID: 26697075
- PMCID: PMC4677248
- DOI: 10.1155/2016/4851081
Human Amnion-Derived Mesenchymal Stem Cells Promote Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells by Influencing the ERK1/2 Signaling Pathway
Abstract
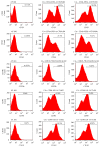
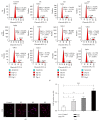
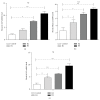
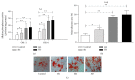
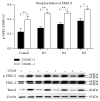
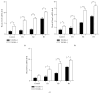
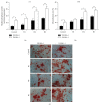
Human amnion-derived mesenchymal stem cells (HAMSCs) are considered to be an important resource in the field of tissue engineering because of their anti-inflammatory properties and fewer ethical issues associated with their use compared with other sources of stem cells. HAMSCs can be obtained from human amniotic membranes, a readily available and abundant tissue. However, the potential of HAMSCs as seed cells for treating bone deficiency is unknown. In this study, HAMSCs were used to promote proliferation and osteoblastic differentiation in human bone marrow mesenchymal stem cells (HBMSCs) in a Transwell coculture system. Proliferation levels were investigated by flow cytometry and immunofluorescence staining of 5-ethynyl-2'-deoxyuridine (EdU). Osteoblastic differentiation and mineralization were evaluated in chromogenic alkaline phosphatase (ALP) activity substrate assays, Alizarin red S staining, and RT-PCR analysis of early HBMSCs osteogenic marker expression. We demonstrated that HAMSCs stimulated increased alkaline phosphatase (ALP) activity, mRNA expression of osteogenic marker genes, and mineralized matrix deposition. Moreover, the effect of HAMSCs was significantly inhibited by U0126, a highly selective inhibitor of extracellular signaling-regulated kinase 1/2 (ERK1/2) signaling. We demonstrate that HAMSCs promote osteogenic differentiation in HBMSCs by influencing the ERK1/2 signaling pathway. These observations confirm the potential of HAMSCs as a seed cell for the treatment of bone deficiency.
Figures

Similar articles
-
Role of human amnion-derived mesenchymal stem cells in promoting osteogenic differentiation by influencing p38 MAPK signaling in lipopolysaccharide -induced human bone marrow mesenchymal stem cells.Exp Cell Res. 2017 Jan 1;350(1):41-49. doi: 10.1016/j.yexcr.2016.11.003. Epub 2016 Nov 8. Exp Cell Res. 2017. PMID: 27832946
-
Human amnion mesenchymal stem cells promote proliferation and osteogenic differentiation in human bone marrow mesenchymal stem cells.J Mol Histol. 2015 Feb;46(1):13-20. doi: 10.1007/s10735-014-9600-5. Epub 2014 Nov 29. J Mol Histol. 2015. PMID: 25432786
-
A comparative study of HAMSCs/HBMSCs transwell and mixed coculture systems.IUBMB Life. 2019 Jul;71(7):1048-1055. doi: 10.1002/iub.2074. Epub 2019 May 21. IUBMB Life. 2019. PMID: 31112365
-
Human Amniotic Mesenchymal Stem Cells Promote Endogenous Bone Regeneration.Front Endocrinol (Lausanne). 2020 Oct 2;11:543623. doi: 10.3389/fendo.2020.543623. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33133012 Free PMC article. Review.
-
Amnion-derived stem cell transplantation: A novel treatment for neurological disorders.Brain Circ. 2016 Jan-Mar;2(1):1-7. doi: 10.4103/2394-8108.178537. Epub 2016 Mar 11. Brain Circ. 2016. PMID: 30276271 Free PMC article. Review.
Cited by
-
Comparable osteogenic capacity of mesenchymal stem or stromal cells derived from human amnion membrane and bone marrow.Cytotechnology. 2018 Apr;70(2):729-739. doi: 10.1007/s10616-017-0177-1. Epub 2018 Jan 5. Cytotechnology. 2018. PMID: 29305674 Free PMC article.
-
FGF-2-Induced Human Amniotic Mesenchymal Stem Cells Seeded on a Human Acellular Amniotic Membrane Scaffold Accelerated Tendon-to-Bone Healing in a Rabbit Extra-Articular Model.Stem Cells Int. 2020 Jan 6;2020:4701476. doi: 10.1155/2020/4701476. eCollection 2020. Stem Cells Int. 2020. PMID: 32399042 Free PMC article.
-
Biomimetic nucleus pulposus scaffold created from bovine caudal intervertebral disc tissue utilizing an optimal decellularization procedure.J Biomed Mater Res A. 2016 Dec;104(12):3093-3106. doi: 10.1002/jbm.a.35858. Epub 2016 Aug 19. J Biomed Mater Res A. 2016. PMID: 27507100 Free PMC article.
-
Human amnion-derived mesenchymal stem cells promote osteogenic and angiogenic differentiation of human adipose-derived stem cells.PLoS One. 2017 Oct 11;12(10):e0186253. doi: 10.1371/journal.pone.0186253. eCollection 2017. PLoS One. 2017. PMID: 29020045 Free PMC article.
-
Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin.Int J Nanomedicine. 2018 Jan 25;13:505-523. doi: 10.2147/IJN.S152105. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29416332 Free PMC article.
References
-
- Bourne G. L. The microscopic anatomy of the human amnion and chorion. American Journal of Obstetrics & Gynecology. 1960;79(6):1070–1073. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

